Translate this page into:
A clinical and mycological study of onychomycosis in HIV infection
2 Department of Pathology, Grant Medical College and Sir JJ Group of Hospitals, Mumbai, India
3 Department of Microbiology, Grant Medical College and Sir JJ Group of Hospitals, Mumbai, India
Correspondence Address:
Amar Surjushe
Department of Dermatology, Venereology and Leprosy, Grant Medical College and Sir J J Group of Hospitals, Mumbai - 400 008
India
| How to cite this article: Surjushe A, Kamath R, Oberai C, Saple D, Thakre M, Dharmshale S, Gohil A. A clinical and mycological study of onychomycosis in HIV infection. Indian J Dermatol Venereol Leprol 2007;73:397-401 |
Abstract
Background: Onychomycosis is one of the early manifestations of HIV infection with a prevalence of 15-40%. Multiple nail involvement, isolation of both common and rare species, and resistance to treatment are the characteristics of onychomycosis in HIV. Aim: To study the epidemiology, clinical manifestations of onychomycosis in HIV-infected individuals and to identify the various causative fungi microbiologically. Methods: A total of 250 HIV infected patients, diagnosed by ELISA, were screened for nail involvement; of which 60 patients i.e., 40 males and 20 females, who had clinically suspected untreated fungal infection were included in this study. Results: Of the 60 respondents, 34 (56.66%) were from the 31-40 years age group. Amongst the 40 males, there were 20 manual laborers and 14 farmers; while 18 of 20 females were housewives. Toenail involvement was seen in 38 patients (63.33%), fingernail in 12 patients (20%) while 10 (16.66%) patients had involvement of both. Twenty eight (46.66%) patients gave history of some trauma, 6 (10%) had diabetes mellitus, and only 1 patient (1.66%) had history of peripheral vascular disease. Nineteen (31.66%) patients had associated tinea pedis, 5 (8.33%) had tinea manuum, 10 (16.66%) had tinea corporis and 7 (11.66%) had tinea cruris. Twenty one (35%) respondents had distal and lateral superficial onychomycosis (DLSO), 5 (8.33%) had proximal subungual onychomycosis (PSO), 1 (1.66%) had superficial white onychomycosis (SWO), while 33 (55%) had total dystrophic onychomycosis (TDO). Fungal elements were demonstrated by KOH mount in 49 patients (81.66%) and growth was seen in 32 (53.33%) cultures. Dermatophytes were isolated in 13 (21.66%) and nondermatophytic molds (NDM) in 19 (31.66%). Out of the 13 positive dermatophyte cultures, Trichophyton rubrum was isolated on 11 and Trichophyton mentagrophytes on 2 cultures. Of the 19 non-dermatophytic cultures, Aspergillus niger was isolated on 3 and Candida spp. on 12 while Cladosporium spp., Scytalidium hyalinum, Penicillium spp., and Gymnoascus dankaliensis on 1 each. Conclusions: Total dystrophic onychomycosis was the most common clinical type and NDM were the predominant causative organisms.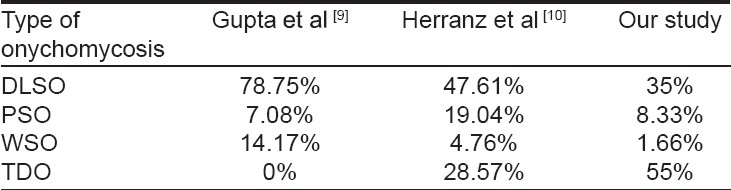

 |
| Figure 5: Summary of mycology results |
 |
| Figure 5: Summary of mycology results |
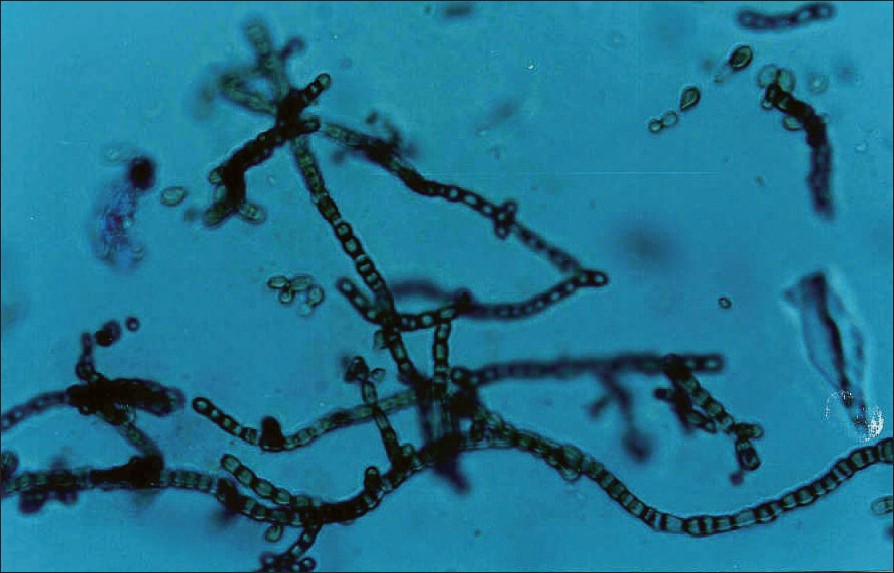 |
| Figure 4: Microscopy of Cladosporium (Lacto phenol cotton blue mount X400) in culture colony showing long branched chains of acropetal one celled, smooth walled lemon shaped conidia |
 |
| Figure 4: Microscopy of Cladosporium (Lacto phenol cotton blue mount X400) in culture colony showing long branched chains of acropetal one celled, smooth walled lemon shaped conidia |
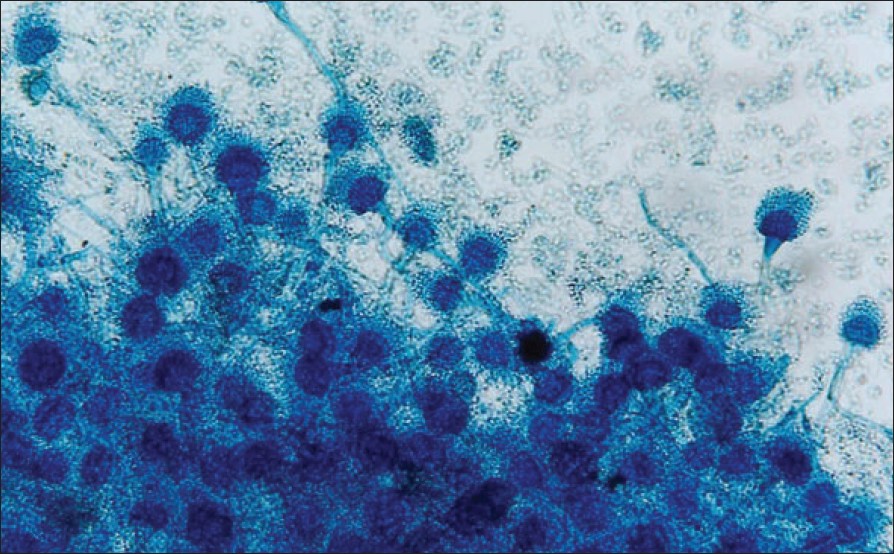 |
| Figure 3: Microscopy of Aspergillus (Lacto phenol cotton blue mount, X400) in culture colony showing vesicles at the apex of the stout conidophore bearing phialides on upper 2/3rd of its surface with chains of small phialoconidia with septate hyphae |
 |
| Figure 3: Microscopy of Aspergillus (Lacto phenol cotton blue mount, X400) in culture colony showing vesicles at the apex of the stout conidophore bearing phialides on upper 2/3rd of its surface with chains of small phialoconidia with septate hyphae |
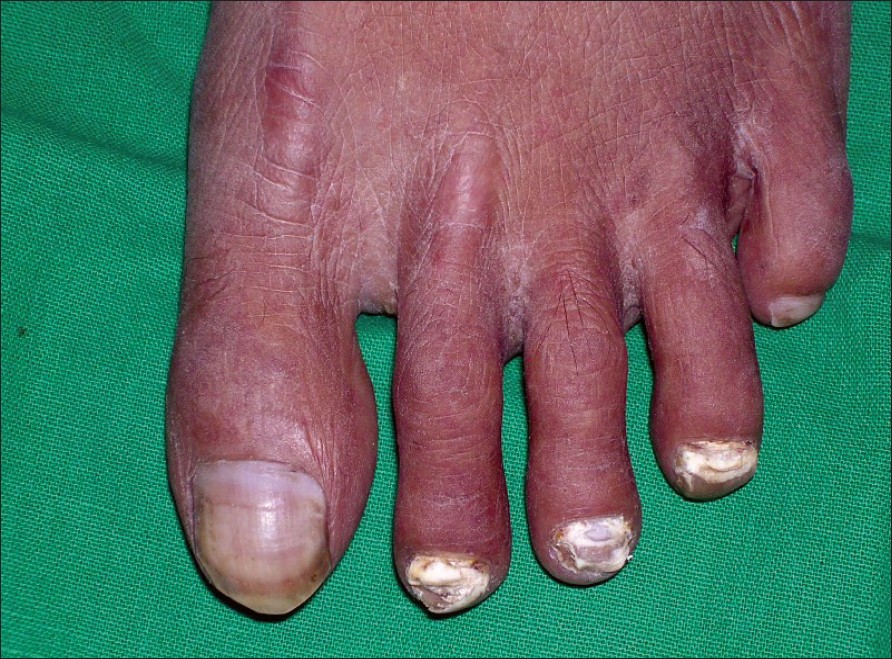 |
| Figure 2: White superfi cial onychomycosis |
 |
| Figure 2: White superfi cial onychomycosis |
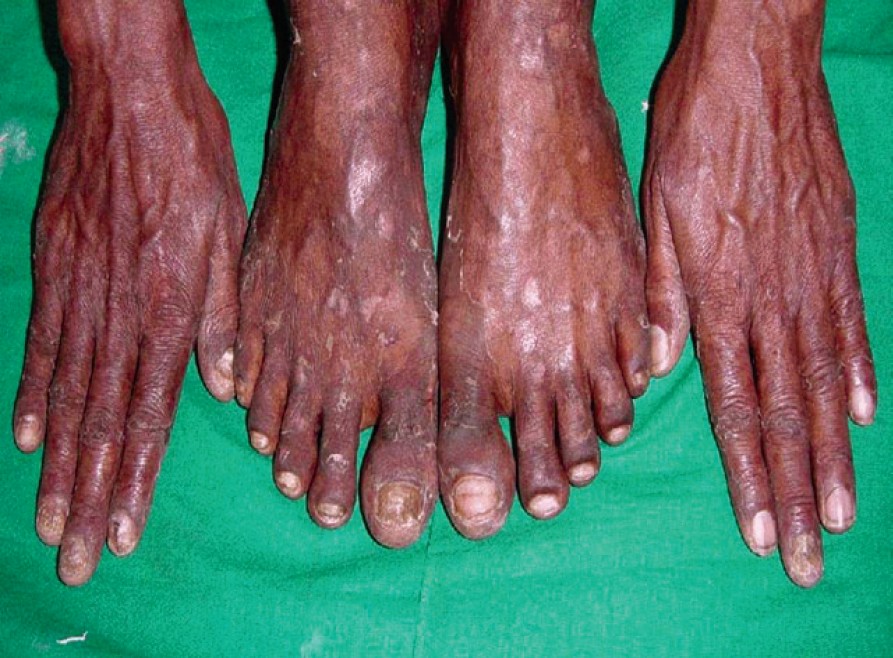 |
| Figure 1: Total dystrophic onychomycosis with tinea pedis and tinea corporis |
 |
| Figure 1: Total dystrophic onychomycosis with tinea pedis and tinea corporis |
Introduction
Onychomycosis associated with acquired immunodeficiency syndrome (AIDS) is characterized by being clinically more aggressive, with a higher frequency of unusual presentations and resistance to conventional treatment. [1] Multiple fungal species and unusual opportunistic fungi are frequently cultured from HIV infected patients and may be a reflection of their immunocompromised state. [2] The prevalence of onychomycosis in HIV infected patients has been reported to be 15-40% [3],[4],[5],[6] and may be directly related to the degree of immunosuppression. [2] It can be an early manifestation of immunosuppression and is more frequent when the CD4 cell count approaches 450 cells/µL. [7] This study is an attempt to elucidate the epidemiology of onychomycosis in HIV infected patients with reference to the prevalence, type and species of the causative fungi with a special reference to the presence of molds.
Methods
A total of 250 HIV infected patients, diagnosed by ELISA, were screened for nail involvement and 60 patients (40 males and 20 females) who had clinically suspected onychomycosis were included in this study. Patients between 20 to 50 years of age who had not received any antifungal treatment were included.
A detailed clinical history was recorded with special emphasis on the type of occupation such as farming, gardening, manual labor etc., and also history of trauma (physical, chemical, mechanical), drug intake or contact with chemicals or cosmetics, diabetes mellitus, peripheral vascular disease and other associated illnesses. Cutaneous examination was carried out with detailed examination of the nail unit. The clinical type of onychomycosis, number of finger or toe nails involved and other associated nail changes such as nail discoloration, subungual hyperkeratosis, crumbling, pitting, thickening, and dystrophy of nail plate were noted.
Sample collection was done by scraping of subungual hyperkeratotic debris and nail plate clipping; depending upon the site and type of involvement and after thorough cleaning of the site with spirit. Direct microscopy was done after overnight incubation of the nail specimen in 20% KOH for the presence of fungal mycelia and spores. All the nail specimens were cultured on Sabouraud dextrose agar (SDA), with chloramphenicol and with or without cycloheximide. Specific medium i.e. Dermatophyte test medium (DTM) was also used for confirmation of dermatophyte infection. Two culture slants were made; one maintained at room temperature and the other was incubated at 37 o C. The cultures were observed twice a week for a period of four weeks and were discarded if there was no growth at the end of 4 weeks. Culture tubes were examined for color of the colony (on the surface and reverse), topography, texture, and rate of growth. In presence of growth, a loopful of growth was taken and examined using a lacto phenol cotton blue (LPCB) mount. Slide culture was also done if required and was examined for characteristic morphology.
Our study design entailed laying down clear criteria for the diagnosis of dermatophyte and non-dermatophyte infection, according to the technical expertise available to us. The criterion used for the diagnosis of dermatophytes was: If a dermatophyte was identified on KOH mount and / or isolated on culture, it was pathogenic.The criteria used for the diagnosis of NDM were: (a) demonstration of fungal filaments, not resembling dermatophyte hyphae and spores on KOH mount, and / or (b) isolation of NDM in pure culture. [8] In our study isolation of NDM on three occasions were considered as pathogenic. The results were recorded and a detailed analysis was done.
Results
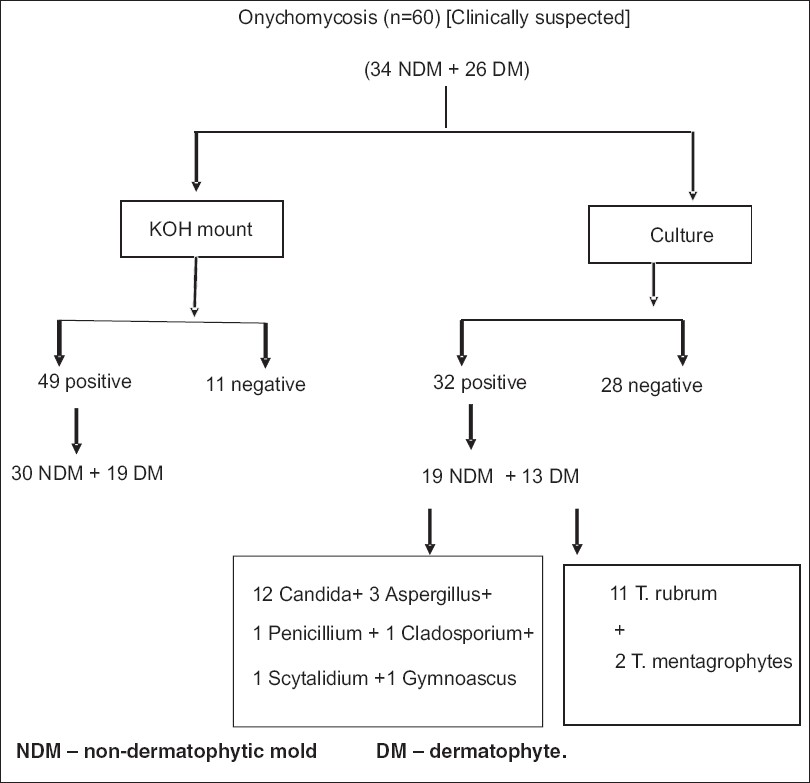
In our study, out of the 60 patients, 16 were between 21-30 years of age (26.66%), 34 were between 31-40 (56.66%) years of age and 10 patients were between 41-50 years of age (16.66%). Out of the 40 male patients, 20 were manual laborers while 14 were farmers. Out of the 20 female cases, 18 were housewives. Toenail involvement was seen in 38 patients (63.33%), fingernail affection in 12 patients (20%) while 10 patients had involvement of both finger nails and toenails (16.66%). Twenty eight patients gave history of trauma (46.66%), 6 patients gave history of diabetes mellitus (10%), and only 1 patient (1.66%) had history of peripheral vascular disease. Nineteen patients had associated tinea pedis (31.66%) [Figure - 1], 5 had tinea manuum (8.33%), 10 had tinea corporis (16.66%) and 7 had tinea cruris (11.66%). Twenty one patients (35%) had DLSO, 5 (8.33%) had PSO, 1 (1.66%) had white superficial onychomycosis [Figure - 2] while the majority i.e. 33 (55%) had TDO. Fungal elements were demonstrated on KOH mounts in 49 patients (81.66%). Fungi were grown on 32 cultures while no fungus was isolated in 28 cultures (46.66%). Of the 32 positive cultures, dermatophytes were isolated in 13 (21.66%) and NDM in 19 (31.66%). Out of the 13 positive dermatophyte cultures, Trichophyton rubrum was grown on 11 and Trichophyton mentagrophytes was grown on 2 cultures. Out of the 19 non-dermatophytic cultures, Candida spp. was isolated on 12, Aspergillus niger on 3 [Figure - 3], Cladosporium spp. on 1 [Figure - 4], Scytalidium hyalinum on 1, Penicillium spp. on 1 and Gymnoascus dankaliensis on 1. The summary of our mycology assays is shown in [Figure - 5].
Discussion
Recently, there has been a spurt in interest regarding onychomycosis in HIV infected patients, but very few at the published studies have been carried out in India. The prevalence of onychomycosis in Canadian and Brazilian studies was found to be 24% (96/400) and 20% (20/100) respectively. [9] Similarly, Cribier et al in France conducted a study between October 1996 and May 1997 and reported a prevalence of 30.32% (47/155). [2] The prevalence in our study was 24% (60/250), which is consistent with the above studies. It has been reported in literature that the prevalence of onychomycosis in HIV infection varies between 15%-40%. [3],[4],[5],[6]
In the present study, the most common age group affected was between 31-40 years, with a mean age of 36.6 years. This corresponds to the findings in Cribier′s (mean age 36.5 ± 8.0 years) [2] and Herranz′s studies (mean age 37 years). [10] This could be due to increased exposure to occupational trauma, and to fungal pathogens with age.
In our study, 40 were males and 20 were females with a sex ratio of 2:1. Thus, males were predominantly involved which is consistent with the findings of Herranz (sex ratio 9.5:1), [10] Ravnborg (sex ratio 2:1), [11] and Gupta (sex ratio 4.88:1). [9] The lower sex ratio in our study is due to a larger sample size and a higher number of female respondents as compared to the studies cited. Out of the 40 males, 20 were laborers while 14 were farmers. Among the females, the majority were housewives i.e. 18/20. Occupations, which are more prone to nail trauma, like manual labor and farming favor the inoculation and growth of fungus, thereby causing onychomycosis. [2] In housewives, frequent contact with water and detergent damages the cuticle, which then favors the invasion by fungi.
Trauma was the major predisposing factor in our study (44.66%), which was followed by diabetes mellitus (10%) and peripheral vascular disease (1.66%). Also, males comprised the larger group of patients who gave a history of trauma. Since in our society, the male still handles the traditional role of the provider, this could make him more liable to trauma. A mechanically damaged nail provides the ideal medium for fungal organisms to invade easily and thrive. Impaired blood supply and associated neuropathy in diabetes favor fungal growth.
Out of the 21 HIV-infected patients of onychomycosis studied by Herraz et al, associated dermatophytosis was present in 7 patients; the majority had tinea pedis (5/21). [10] In our study associated dermatophytosis was noted in 41 out of the 60 patients enrolled. Tinea pedis was the most common finding, present in 19 patients (31.66%), which is consistent with the Herranz study. [10] Existing dermatophytic infection, particularly tinea pedis favours the invasion of nail by the same fungus.
Out of the 22 patients studied by Ravnborg et al , 21 had toenail and 1 had fingernail involvement. [11] In Cribier′s study, out of the 47 patients, 42 had toenail, 3 had fingernail and 2 had both fingernail and toenail involvement, [2] while all the 21 patients in Herranz′s series had toenail involvement, with one individual having infection of fingernails as well. [10] In our study, out of the 60 patients, 38 patients had toenail, 12 had fingernail and 10 patients had both fingernail and toenail involvement. Thus, toenails were more commonly affected. This finding is consistent with all the studies cited above. The predominant involvement of the toenail could be because of greater susceptibility of the toenail to trauma with subsequent invasion by the fungus.
In our study, TDO constituted the majority of 55%, DLSO was seen in 35%, WSO was seen in 1.66 %, and PSO in 8.33%. Total dystrophic onychomycosis was observed in most of our patients. This is at odds with earlier studies in which PSO [12],[13] and DLSO [9],[10] constituted the predominant variety [Table - 1]. The more vigorous nature of the Indian lifestyle could be the probable cause of this discrepancy. A large proportion of our respondents were engaged in manual labor that entails a higher risk of trauma to the nail, and consequently to dystrophic onychomycosis. Also, since total dystrophic onychomycosis is the end result of evolution of any of the other clinical forms, our cases might be the late presentation of the disease. Furthermore, HIV induced immunosuppression may enable the fungus to invade the entire nail and cause dystrophy.
In the study conducted by Ravnborg et al, out of the 22 cultures dermatophytes were isolated in 12 (57.54%) and NDM was isolated in only 1 culture. [11] Cribier reported dermatophyte isolation in 12 (28.57%) and NDM isolation in 15 (35.71%) cultures. [2] In our study out of the 60 cultures, dermatophytes were isolated in 13 (21.66%) and NDM were isolated in 19 cultures (31.66%).
In the cases wherein dermatophytes were isolated, Trichophyton rubrum constituted the majority i.e. 11 followed by Trichophyton mentagrophytes , which was found in 2. Since 19 patients had concurrent tinea pedis, the causative fungi in these patients can be explained.
Among the NDM isolated, 12 were Candida spp., 3 Aspergillus niger, [Figure - 1] 1 Cladosporium spp., [Figure - 2] 1 Scytalidium hyalinum , 1 Penicillium spp. and 1 Gymnoascus dankaliensis [Figure - 5]. Thus NDM were isolated in majority as compared to dermatophytes, which is consistent with Cribier′s findings. This could be because of: A) Increased susceptibility to non-dermatophyte infection in HIV-infected patients due to immunosuppression. B) Environmental factors that favor the growth of non-dermatophytes. C) The ubiquity of a large and varied species of fungi in our environment, as well as the active nature of our lifestyles, which increases the vulnerability to trauma, may be probable causes. The role of Aspergillus spp. as pathogens has been a topic of controversy as they are commonly considered as contaminants. However, various recent studies and case reports have confirmed its pathogenic role. Grover reported Aspergillus niger as a causative pathogen of onychomycosis in 8 patients. [14] Veer et al also reported 50% Aspergillus spp. isolation out of total 13.63% NDM fungal isolates. [15] Other Aspergillus spp. like Aspergillus sclerotiorum, [16] Aspergillus flavus, [17] and Aspergillus tamarii [18] have also been reported as a pathogenic NDM in onychomycosis.
The limitations of this study were as follows. The criteria used for diagnosis of the pathogenic fungi i.e. dermatophytes, nondermatophytic molds or yeast were modified by us according to the microbiologic / mycologic expertise available to us. However, they were stringent and as per accepted norms. Besides, CD4 counts were not performed in our patients because of financial constraints. Hence, we were unable to correlate the type of onychomycosis and causative species with the degree of immunosuppression.
Onychomycosis in HIV patients shows much diversity as compared to non-HIV patients. There are not only differences in the clinical type but also in the causative organisms. This could be because of the immunosuppression in HIV-infected patients, which predisposes this population to uncommon infections. Our study points towards the possibility of NDM being a common cause of onychomycosis, and the consequent need to modify treatment regimens accordingly.
| 1. |
Daniel CR 3 rd , Norton LA, Scher RK. The spectrum of nail disease in patients with human immunodeficiency virus infection. J Am Acad Dermatol 1992;27:93-97.
[Google Scholar]
|
| 2. |
Cribier B, Mena ML, Rey D, Partisani M, Fabien V, Lang J-M, et al. Nail changes in patients infected with human immunodeficiency virus. A prospective controlled study. Arch Dermatol 1998;134:1216-1220.
[Google Scholar]
|
| 3. |
Goodman DS, Teplitz ED, Wishner A, Klein RS, Burk PG, Hershenbaum E. Prevalence of cutaneous disease in patients with acquired immunodeficiency syndrome (AIDS) or AIDS related complex. J Am Acad Dermatol 1987;17:210-20.
[Google Scholar]
|
| 4. |
Matis WL, Triana A., Shapiro R, Eldred L, Polk BF, Hood AF. Dermatologic findings associated with human immunodeficiency virus infection. J Am Acad Dermatol 1987;17:746 - 751.
[Google Scholar]
|
| 5. |
Kaplan MH, Sadick N, McNutt NS, Meltzer M, Sarngadharan MG, Pahwa S. Dermatologic findings and manifestations of acquired immunodeficiency syndrome (AIDS). J Am Acad Dermatol 1987;16:485-506.
[Google Scholar]
|
| 6. |
Prose NS, Abson KG, Scher RK. Disorders of the nails and hair associated with human immunodeficiency virus infection. Int J Dermatol 1992:31;453-457.
[Google Scholar]
|
| 7. |
Conant MA. The AIDS epidemic. J Am Acad Dermatol 1994;31:47-50.
[Google Scholar]
|
| 8. |
English MP. Nails and fungi. Br J Dermatol 1976;94:697-701.
[Google Scholar]
|
| 9. |
Gupta AK, Taborda P, Taborda V, Gilmour J, Rachlis A, Salit I, et al . Epidemiology and prevalence of onychomycosis in HIV-positive individuals. Int J Dermatol 2000;39:746-53.
[Google Scholar]
|
| 10. |
Herranz P, Garcia J, De Lucas R, Gonzalez J, Pena JM, Diaz R, et al. Toenail onychomycosis in patients with acquired immune deficiency syndrome: treatment with terbinafine. Br J Dermatol 1997;137:577-580.
[Google Scholar]
|
| 11. |
Ravnborg L, Baastrup N, Svejgaard E. Onychomycosis in HIV-infected patients. Acta Derm Venereol 1998;78:151-2.
[Google Scholar]
|
| 12. |
Elewski BE: Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-1.
[Google Scholar]
|
| 13. |
Dompmartin D, Dompmartin A, Deluol AM, Grosshans E, Coulaud JP. Onychomycosis and AIDS: clinical and laboratory findings in 62 patients. Int J Dermatol 1990;29:337-339.
[Google Scholar]
|
| 14. |
Grover S. Clinicomycological evaluation of onychomycosis at Banglore and Jorhat. Indian J Dermatol Venerol Leprol 2003;69:284-6.
[Google Scholar]
|
| 15. |
Veer P, Patwardhan NS, Damle AS. Study of onychomycosis: Prevailing fungi and pattern of infection. Indian J Med Microbiol 2007;25:53-56.
[Google Scholar]
|
| 16. |
Singh SM, Barde AK. A case of onychomycosis caused by Aspergillus sclerotiorum . Indian J Dermatol Venerol Leprol 1983;49:22-5.
[Google Scholar]
|
| 17. |
Mahmoudabadi AZ, Zarrin M. Onychomycosis with Aspergillus flavus: a case report from Iran. Pak J Med Sci 2005;21:497-498.
[Google Scholar]
|
| 18. |
Kristensen L, Stenderup J, Otkjζr A. Onychomycosis due to Aspergillus tamarii in a 3-year-old boy. Acta Dermato-Venereologica 2005;85:261-262.
[Google Scholar]
|
Fulltext Views
5,716
PDF downloads
2,437





