Translate this page into:
S�zary cell
Correspondence Address:
M J Cyriac
Department of Dermatology and Venereology, Medical College, Kottayam, Kerala - 686 008
India
| How to cite this article: Cyriac M J, Kurian A. S�zary cell. Indian J Dermatol Venereol Leprol 2004;70:321-324 |

 |
 |
 |
 |
Sézary cells (SC) (synonyms: mycosis cell, Lutzner cell, cerebriform lymphocytes, Atypia Cellules Monstreuses or monster cell[1],[2] are atypical lymphocytes with a grooved or cerebriform nucleus seen both in tissue and blood. They are characteristically seen in cutaneous T cell lymphomas including mycosis fungoides (MF). The Sézary cell is named after the French dermatologist Sézary A (1880-1956).[3] Sézary and Bouvrain described the leukemic variant of MF in 1938 where they identified the monster cells with atypical features. This variant is now known as Sézary syndrome. In 1968, Lutzner and Jordan[4] described the ultrastructural details of Sézary cells.
MORPHOLOGY
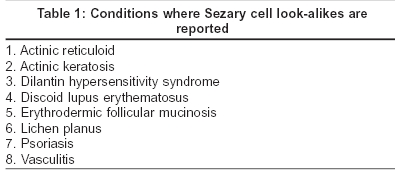
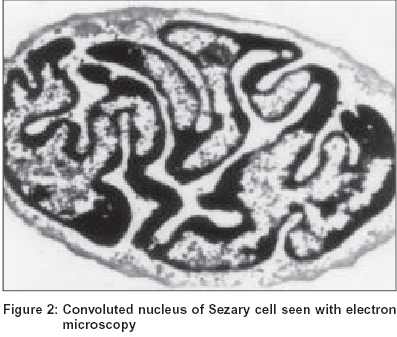
Three variants of Sézary cell are identified based on its size. The ′small cells′ are less than 12 microns in diameter, ′large cells′ are more than 12 microns and the ′very large cells′ are more than 14 microns.[5] The nucleus, occupying at least four fifths of the cell, shows a variable surface contour ranging from mild indentations to gross cerebriform shape [Figure - 1], [Figure - 2].[6] The nuclear chromatin is condensed in a patchy distribution beneath the nuclear membrane. The nucleoli may be prominent. The cytoplasm is sparse and appears as a narrow rim around the nucleus. Mitochondria, often appearing clumped, rough endoplasmic reticulum, polysomes and cytoplasmic fibrils are seen. The cytoplasmic fibrils appear serpentine on electron microscopic examination.[7]
IMMUNOHISTOCHEMISTRY
Sézary cell is a malignant CD4+ helper T cell of the T helper 2 subset.[8] It produces cytokines (IL-4, IL-5 and IL-10) that enhance the differentiation and activation of eosinophils and suppress T helper 1 activity. The early lesion of mycosis fungoides retains the expression of both majority T cell markers like CD7 and Leu-8 (CD62L) which are present on most mature non-neoplastic T cells and pan T cells markers like CD2,CD3 and CD5 which are present on all mature non-neoplastic T cells. As lesions progress the earliest marker lost is a majority T cell antigen. Majority T cell markers may be lost in both MF and in 10-20% of inflammatory dermatoses. Pan T cell markers are less commonly lost in MF, but if they are lost then we can exclude the possibility of it being an inflammatory dermatosis. The absence of CD7 or Leu-8 within epidermis has been observed in mycosis fungoides, a feature absent in inflammatory lesions.[9],[10],[11],[12]
Dipeptidyl peptidase IV (CD26) is found to be absent on circulating neoplastic cells in Sézary syndrome. The level of CD4+ CD26- T cell subpopulation correlates with the extent of peripheral blood involvement.[13] Recently CD158k marker has been demonstrated on Sézary cells. This marker may prove useful as a tool for evaluation of circulating tumoral burden and in the follow up of patients with Sézary syndrome.
HISTOGENESIS
The site of origin of Sézary cell has not been determined. Large numbers of these cells are seen in the dermis in MF and Sézary syndrome. Epidermotropism is noted more markedly in mycosis fungoides as compared to Sézary syndrome. It has been shown that these atypical cells are in fact actively dividing in the dermis itself, the dermis forming a home to these cells. The cells appearing in the peripheral blood are probably an overspill phenomenon. The observations that these cells cannot be demonstrated in abnormal amounts in bone marrow and that they tend to disappear when skin lesions resolve support this theory.
It is believed that the evolution of mycosis fungoides starts with the aggregation of circulating T helper cells in the papillary dermis. This is followed by a selection phase where cells with a T helper 2 cytokine profile predominate and are then transformed into a clonal population by various cytokines. The final stage is the dissemination phase in which the neoplastic cells dedifferentiate without the need of the skin microenvironment. The critical step in the cascade seems to be the commitment to the T helper 2 subtype. Once switched to the T helper 2 profile the cytokines produced suppress immunosurveillance by the cytotoxic T lymphocytes and the natural killer cells allowing for unremitting stimulation of the T helper cells by the autocrine loop and by antigen presenting cells.
A higher incidence of Sézary syndrome is seen with occupational exposure to industrial solvents and in those employed in the petrochemical, textile, manufacturing and construction industries. Recently, the Human T cell lymphotropic virus I /II have been implicated although molecular analyses have been inconclusive.
LABORATORY DIAGNOSIS
In blood smear Sézary cells appear as mononuclear cells with grooved nuclei and cytoplasmic vacuoles, which can often be stained with Periodic Acid Schiff reagent. Glycogen is responsible for staining in some, but not in all of the vacuoles. These cells can also be recognized by Giemsa or Wright staining methods. Some Sézary cells are the size of normal lymphocyte while others are much larger. Based on the cytomorphology Sézary syndrome has been classified by the International Society for Cutaneous Lymphomas (ISCL) as:
1. Small cell variant: more than 80% of SC are small cells
2. Large cell variant: more than 20% of SC are very large cells
3. Mixed cell variant: an intermediate number of large SC and small SC
Patient with very large SC in the blood have a worse prognosis that those without. Smears are not a particularly sensitive method of quantifying the number present. It must be realized that it is not necessary to have a raised total lymphocyte count for the diagnosis to be made and in such situations unless the tail of the smear or the buffy coat is specifically examined for the atypical cells, the diagnosis may easily be missed. At least 100 lymphocytes are counted to determine the percentage of cells showing Sézary phenomenon. Lutzner, et al compared the ultrastructure of the abnormal cells in the skin, lymph nodes and peripheral blood of patients with Sézary syndrome and found that the cells were similar. Ultrastructural morphometry has been used to distinguish Sézary cells in blood from normal lymphocytes and reactive lymphocytes.
CLINICAL SIGNIFICANCE
In mycosis fungoides the Sézary cell is considered malignant based on following criteria:
1. They are present in skin lesions and viscera
2. Have abnormal number of chromosomes and abnormal karyotypes including marker chromosomes
Diagnosis of Sézary syndrome as originally described requires the presence of the triad of erythroderma, lymphadenopathy and 10% or more of atypical mononuclear cells in the peripheral smear. Many experts now consider a circulating Sézary cell count which exceeds 1000 cells/cumm as characteristic of Sézary syndrome and the prognosis to be worse in patients with circulating Sézary cell counts of> 5% of total lymphocyte count. The term pre Sézary syndrome is used if cell count is < 1000 cells/cumm with erythroderma, although others believe that presence of Sézary cell by itself is significant irrespective of the number.
Blood criteria to define Sézary syndrome as recently proposed by ISCL are the following:
- An absolute Sézary cell count of 1000 cells/cumm or more
- An increase in CD3 or CD4 positive cells resulting in a CD4/CD8 ratio of 10 or more
- Aberrant expression of pan T cell markers by flow cytometry, deficient CD7 expression
- Increased relative or absolute lymphocyte counts with evidence of a T cell clone in the blood by Southern blot or PCR technique
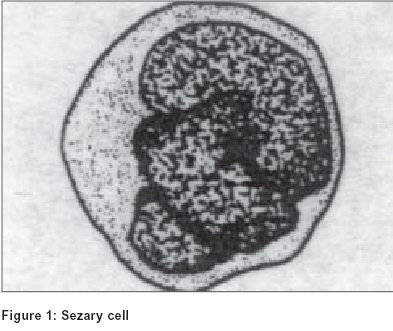
Although Sézary cell is the pathological hallmark of cutaneous T cell lymphoma, its significance has been brought into dispute as similar atypical mononuclear cells have been described in a variety of other skin disorders [Table - 1]. The conditions include lichen planus, discoid lupus erythematosus, psoriasis, vasculitis, actinic keratosis,[6] actinic reticuloid, dilantin hypersensitivity syndrome and erythrodermic follicular mucinosis. Whether CD4+ CD7- T cells present in the epidermis and the dermis represent the neoplastically transformed cell population can be determined using the panel of antibodies to the variable regions of T cell receptor (TCR beta chain).[9] Although chromosomal characteristics/DNA content of the Sézary look-alike cells in benign dermatoses is yet to be studied, such studies of Sézary cell reveal abnormal number of chromosomes and abnormal karyotypes pointing towards its malignant nature.
| 1. |
Goldberg DJ, Schinella RS, Kechijian P. Hypopigmented mycosis fungoides-speculations about the mechanisms of hypopigmentation. Am J Dermatopathol 1986;8:326-30.
[Google Scholar]
|
| 2. |
Van der Putte SC, Van der Meer JB.Mycosis fungoides-a morphological study. Clin Exp Dermatol 1981;6:57-76.
[Google Scholar]
|
| 3. |
Willemze R. Diagnostic criteria in S�zary syndrome. J Invest Dermatol 1983;81:392-7.
[Google Scholar]
|
| 4. |
Lutzner MA, Jordan HW. The ultrastructure of an abnormal cell in S�zary syndrome. Blood 1968;31:719.
[Google Scholar]
|
| 5. |
Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroche L, et al. Update on erythrodermic cutaneous T cell lymphoma: Report of the international Society for cutaneous lymphoma. J Am Acad Dermatol 2002;46:95-106.
[Google Scholar]
|
| 6. |
Heald PW, Edelson RL. Cutaneous T cell lymphoma. In: Freedberg IM, Eisen AF, Wolff K, Austen KF, Goldsmith LA, Katz SI, et al. editors. Fitzpatrick's Dermatology in General Medicine. 5th Ed. New York: McGraw Hill; 1999. p. 1227-50.
[Google Scholar]
|
| 7. |
Le Boit PE, McCalmont TH. Cutaneous Lymphoma and leukemia. In: Elder D, Elenitsas R, Jaworsky C, editors. Lever's Histopathology of the skin. 8th Ed. New York: Lippincott Raven; 1999. p. 805-46.
[Google Scholar]
|
| 8. |
Suchin KR, Cassin M, Gottlib SL, Sood S, Cucchiara AJ, Vondeiheid EC, et al. Increased interleukin 5 production in eosinophilic S�zary syndrome. Regulation by IFN-� & IL-12. J Am Acad Dermatol 2001;44:28-32.
[Google Scholar]
|
| 9. |
Rappl G, Muche JM, Abken H, Sterry W, Jilgen W, Urgel S. CD4+CD7- cells compose the dominant T cell clone in the peripheral blood of the patients with S�zary syndrome. J Am Acad Dermatol 2001;44:456-61.
[Google Scholar]
|
| 10. |
Wood GS, Abel EA, Hoppe RT. Leu-8 and Leu-9 antigen phenotypes-Immunologic criteria for the distinction of mycosis fungoides from cutaneous inflammations. J Am Acad Dermatol 1986;14:1006-13.
[Google Scholar]
|
| 11. |
Yoo EK, Cassin M, Lessin SR, Rook AH. Complete molecular remission during biologic response modifier therapy for S�zary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity. J Am Acad Dermatol 2001;45:208-16.
[Google Scholar]
|
| 12. |
Jalpur R, Lifshitz O, Mc Ham JB, Duvic M. Increased serum immunoglobulin levels are common in mycosis fungoides and S�zary syndrome. J Am Acad Dermatol 2002;47:685-91.
[Google Scholar]
|
| 13. |
Bernengo MG, Novelli M, Quanglino P. The relevance of the CD4+CD26- subset in the identification of circulating S�zary cells. Br J Dermatol 2001;144:125-35.
[Google Scholar]
|
Fulltext Views
13,539
PDF downloads
3,903





