Translate this page into:
Correlation of the severity of atopic dermatitis with absolute eosinophil counts in peripheral blood and serum IgE levels
2 Departments of Pediatrics, Institute of Child Health, Kolkata, India
3 Department of Pathology, AMRI-Apollo Hospitals, Kolkata, India
Correspondence Address:
Sandipan Dhar
Flat 2A2, Block 2, 5, N.S.C. Bose Road, Kolkata - 700 040
India
| How to cite this article: Dhar S, Malakar R, Chattopadhyay S, Dhar S, Banerjee R, Ghosh A. Correlation of the severity of atopic dermatitis with absolute eosinophil counts in peripheral blood and serum IgE levels. Indian J Dermatol Venereol Leprol 2005;71:246-249 |
Abstract
BACKGROUND: Although a number of epidemiological studies, showing incidence and prevalence of atopic dermatitis, were available, scant attention has been paid to the correlation between the parameters of the disease like severity, absolute eosinophil count and IgE level, which has been known to be associated inconsistently. Hence this study was undertaken. METHODS: A total of 102 patients of atopic dermatitis, both children and adults, and 107 age matched controls were studied at the Pediatric Dermatology clinic, Institute of Child Health and department of Dermatology, AMRI-Apollo hospitals, Kolkata. RESULTS: The average age of onset of atopic dermatitis was observed to be 4.55 years. Both the average absolute eosinophil count and IgE levels in patients of atopic dermatitis were significantly higher than that of the controls. Each of these parameters showed significant correlation with severity of the disease and showed a nonhomogeneous distribution reflected by significant association with personal history of bronchial asthma and family history of atopy, when both parents were atopic. CONCLUSIONS: Our study shows that clinical activity of the disease as recorded by the "SCORAD" index can be used as an indicator of the hematological abnormalities as well as to some extent as a prognostic indicator. Family history of atopy correlates with the hematological abnormalities only if both parents are involved and bronchial asthma is the only associated atopic condition which correlates with the parameters of the disease .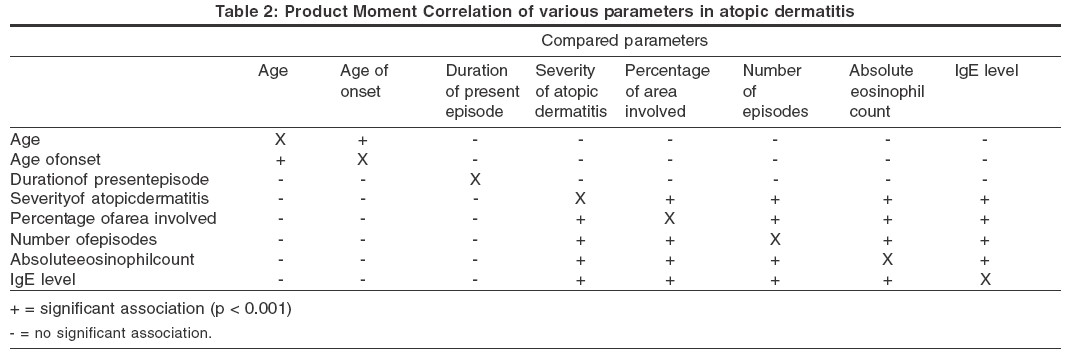



INTRODUCTION
Atopic dermatitis (AD) is an itchy, chronic, or chronically relapsing inflammatory skin condition. The rash is characterized by itchy papules (occasionally vesicles in infants) that become excoriated and lichenified, and typically have a flexural distribution. The eruption is frequently associated with other atopic conditions in the individual or in other family members. [1],[2],[3],[4] The main immunological abnormalities are excessive formation of IgE, with a predisposition to anaphylactic sensitivity, some decrease in susceptibility to delayed hypersensitivity, abnormalities in expression of surface molecules in antigen presenting cells, and dysregulation of cytokine mediators. The severity of AD has some positive correlation with the absolute eosinophil count and serum IgE levels,[2],[3] but this is not a consistent observation. Hence, we performed a study to elucidate the correlation between the severity of AD, the absolute eosinophil count in the blood and the serum IgE levels. To the best of our knowledge, this is the first study of its kind from the Indian subcontinent.
MATERIALS AND METHODS
The study was carried out at the Pediatric Dermatology Clinic, Institute of Child Health and Department of Dermatology, AMRI-Apollo hospitals, Kolkata. A total of 102 consecutive patients, both children and adults, with atopic dermatitis attending the hospitals were enrolled. One hundred and seven age- and sex-matched persons without any personal or family history of atopy were taken as controls. Patients with atopic dermatitis having other systemic diseases were excluded from the study.
For every patient a detailed history was taken, including the age at onset, duration of present illness, personal and/or family history of atopic disorder, and this was recorded in a pro-forma. Written consent was obtained from the subjects or their parents before enrollment.
A thorough clinical examination was carried out to determine the surface area of involvement of the disease (using Wallace′s Rule of Nine) and the severity (using the SCORAD index).
The SCORAD index is a scoring system designed by the European Task Force on Atopic Dermatitis to measure the severity of atopic dermatitis. It has five clinical signs, viz. erythema, vesiculation, excoriation, crusting and edema, each of these signs with four scores: 0 = absent; 1 = mild, 2 = moderate, and 3 = severe. The total IgE level and absolute eosinophil count were obtained in each patient.
One way analysis of variance was performed on parameters of severity of atopic dermatitis, eosinophil count and IgE levels with respect to independent variables like sex, family history of atopy (father, mother or both), sex and associated atopic conditions (bronchial asthma, allergic rhinitis). Each of the parameters was compared with each other using Product Moment Correlation to observe any significant covariance.
RESULTS
The baseline demography of the cases and the controls are shown in [Table - 1].
The age of onset of the disease ranged from 6 months to17.5 years, with a mean of 4.55 years (SD 3.63). The severity of atopic dermatitis, as recorded using the SCORAD index, ranged from 10 (maximum possible value, 15) to 1 (minimum possible value, 0), with a mean of 5.48 (SD 2.59). The mean percentage of body surface area involved was 7.07 (SD 5.13). The mean of the total number of attacks of the disease suffered by the patient before presentation was 6 (SD 4.65) and that of the duration of present illness was 3.02 months (SD 1.78). The absolute eosinophil count ranged from 34 to 3453, with a mean of 624 (SD 590), in patients with atopic dermatitis, and from 10 to 512 with a mean of 105 (SD 83) in controls. Serum IgE levels ranged from 22 to 1188 IU, with a mean of 278.2 (SD 324.85), in patients, and from 2 to 126 IU, with a mean of 25.8 IU (SD 23.36), in controls.
The results of the Product Moment Correlation are shown in [Table - 2]. Product Moment Correlation showed that there was significant (p< 0.0001) covariance between the severity of atopic dermatitis, total number of episodes, absolute eosinophil count and IgE level. Three parameters, the severity of atopic dermatitis, the absolute eosinophil count and the IgE level, individually showed significant association with a family history of atopy only if both parents were atopic. These parameters also showed a significant association with a history of bronchial asthma in patients of atopic dermatitis but not with a history of allergic rhinitis. The age of onset of the disease was not significantly associated with a personal or a family history of atopy.
One-way analysis of variance performed on the IgE level with respect to the month of estimation showed no significant association. The duration of the disease or the chronicity of the eczema did not correlate with IgE levels and eosinophil count.
DISCUSSION
Hospital based studies suggest that the age of onset of AD is under 6 months in 75% of the cases and before the age of 5 years in 80% to 90%, but occasionally onset may be delayed until later childhood or adult life. [5],[6],[7] In Singapore, a retrospective analysis showed that the onset was before the age of 10 years in 61.2% cases and in 13.6% cases after the age of 21years.[8] In a study from northern India, the age of onset was separately obtained for infantile AD and childhood AD. The mean age of onset in the infantile group was 4.3 months, and that in the childhood group was 4.1 years.[9] We found that the mean age at onset of AD was 4.55 (SD 3.63) years, an observation similar to that of childhood AD in the earlier Indian study.
We also found that in patients with AD the mean absolute eosinophil count was 624 (SD 590) and the mean IgE level was 278.29 (SD 324.85); the corresponding values were 121 (SD 109) and 25.8 (SD 23.36) respectively for controls. Thus, the absolute eosinophil count and the IgE level were significantly higher in patients with AD than in controls.
A Japanese study found that the eosinophil levels roughly correlated with the disease severity, but the pattern of eosinophilia was not homogeneous.[10] Very high eosinophil counts were common in severe cases of AD who had a personal or family history of respiratory atopy, while normal or moderately elevated counts were obtained in severe cases of ′pure′ atopic dermatitis who had neither a personal nor a family history of respiratory atopy. It was suggested that disease severity and personal or family history of respiratory atopy are important factors in determining high blood eosinophil levels in atopic dermatitis.[10] Similarly, we found that both the absolute eosinophil count and the IgE level showed significant covariance with disease severity. The non-homogeneous distribution of the absolute eosinophil count and the IgE level were reflected in the large range and higher standard deviation. One way analysis of variance showed a significant association of the absolute eosinophil count and the IgE level with a family history of AD only when both parents were affected. The eosinophil count and the IgE level also showed a significant association with a history of bronchial asthma in patients with AD but not with allergic rhinitis.
A Japanese study evaluated the role of eosinophils in atopic dermatitis by correlating levels of serum eosinophil cationic protein (ECP), clinical activity, eosinophil count and IgE level. [11] A positive correlation was observed between the number of peripheral blood eosinophils and serum ECP levels in severe cases (r = 0.67, p < 0.05). Further, the ECP levels significantly decreased with clinical improvement of AD or decreased numbers of blood hypo-dense eosinophils in anti-allergic drug-treated patients. There was no correlation between serum ECP and IgE levels. These findings indicate that eosinophils may release their granular contents, including ECP, into the peripheral circulation and/or inflammatory skin lesions and subsequently provoke a clinical exacerbation by stimulating allergic reactions.[11] Our study not only show similar results but also highlights a significant association of clinical activity (severity) of AD with IgE levels in the blood.
The age at onset of AD did not show any significant association with associated atopic conditions or with a family history of atopy.
In atopic dermatitis, a Th1/Th2 imbalance has been reported, and interleukin (IL)-13 seems to play a pivotal role in the inflammatory network. Grutta et al assessed the correlation between the immunological marker CD4 (+) cells, interleukin (IL)-13 (+) and the clinical phase of extrinsic AD in children.[12] They found that blood CD4 (+) cells, IL-4 (+) were significantly higher in the AD group than in controls. CD4 (+) IL-13 (+) cells in the AD group correlated well (p = 0.0007) with the SCORAD index. At remission, there was a significant correlation between SCORAD index and eosinophil count (p < 0.03) and the percentage of CD4 (+) IL-13 (+) cells globally decreased (p < 0.0001), while no difference was found among SCORAD classes. This study confirmed the Th2 profile predominance in the peripheral blood of children with AD, and the close relationship between the number of CD4 (+) IL-13 (+) T cells and disease severity.[12]
The elevated IgE response and eosinophilia observed in patients with atopic dermatitis (AD) may reflect increased responses of type 2 T-helper (Th2) cytokines with a concomitant decrease in interferon-gamma (IFN-gamma) production. However, the cross-regulation of Th1/Th2 derivation and function in AD patients are incompletely characterized. Yoshizawa et al investigated serum levels of several cytokines (IL-18, IL-12, IL-10, IL-2 and IFN-gamma) in patients with AD to assess their possible relationship to the severity of disease. Serum IL-18 levels in AD patients were significantly higher than in healthy controls and significantly correlated with eosinophil counts and serum soluble IL-2 receptor (sIL-2R) levels. They showed a tendency to correlate with clinical severity scores and serum IgE levels. IL-2 levels showed a significantly inverse correlation with serum IgE levels, and IL-12 levels clearly correlated with IL-10 levels. These results suggest the value of serum IL-18 levels as a parameter of AD activity and may support a possible role for IL-18 in the pathogenesis of AD. The inverse correlation between IgE levels and IL-2 levels suggests that IgE production may be inhibited by IL-2 in patients with AD.[13] Our study shows that the clinical activity of AD, as recorded by the SCORAD index, can be used as an indicator of the hematological abnormalities as well as, to some extent, a prognostic indicator in the majority of patients. Family history of atopy is important only if both parents are affected and bronchial asthma is the only associated atopic condition that correlates with the various parameters of the disease.
| 1. |
Ohman S, Johansson SG .Immunoglobins in atopic dermatitis, with special reference to IgE. Acta Derm Venereol 1974;54:193-202.
[Google Scholar]
|
| 2. |
Ohman S, Johansson SG. Allergen-specific IgE in atopic dermatitis. Acta Derm Venereol 1974;54:283-90.
[Google Scholar]
|
| 3. |
Jones HE, Inouye JC, McGerity JL, Lewis CW. Atopic disease and serum immunoglobin-E. Br J Dermatol 1975;92:17-25.
[Google Scholar]
|
| 4. |
Uehara M. Family background of respiratory atopy: a factor of serum IgE elevation in atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:78-82
[Google Scholar]
|
| 5. |
Musgrove K, Morgan JK. Infantile eczema: a long- term follow up study. Br J Drematol 1976;95:365-72.
[Google Scholar]
|
| 6. |
Rajka G. Essential aspect of atopic dermatitis. Berlin: Springer-Verlag; 1989.
[Google Scholar]
|
| 7. |
Vickors CF. The natural history of atopic eczema. Acta Derm Venereol Suppl (Stockh) 1980;92:113-5.
[Google Scholar]
|
| 8. |
Tay YK, Khoo BP, Gol CL. The epidemiology of atopic dermatitis at a tertiary referral skin center in Singapore. Asian Pac J Allergy Immunol 1999;17:137-41.
[Google Scholar]
|
| 9. |
Dhar S, Kanwar AJ. Epidemiology and clinical pattern of atopic dermatitis in a north Indian pediatric population. Pediatric Dermatol 1998;15:347-51.
[Google Scholar]
|
| 10. |
Uehara M, Izukura R, Sawai T. Blood eosinophilia in atopic dermatitis. Clin Exp Dermaotol 1990;15:264-6.
[Google Scholar]
|
| 11. |
Tsuda S, Kato k, Miyasto M, Sasai Y. Eosinophil involvement in atopic dermatitis as reflected by elevated serum levels of eosinophil cationic protein. J Dermatol 1992;19:208-13.
[Google Scholar]
|
| 12. |
La Grutta S, Richiusa P, Pizzolanti G, Mattina A, Pajno GB, Citarrella R, et al . CD4(+)IL-13(+) cells in peripheral blood well correlates with the severity of atopic dermatitis in children. Allergy 2005;60:391-5.
[Google Scholar]
|
| 13. |
Yoshizawa Y, Nomaguchi H, Izaki S, Kitamura K. Serum cytokine levels in atopic dermatitis. Clin Exp Dermatol 2002;27:225-9.
[Google Scholar]
|
Fulltext Views
6,016
PDF downloads
2,148





