Translate this page into:
Different progression pattern between acral and nonacral melanoma: A retrospective, comparative, clinicoprognostic study of 492 cases of primary cutaneous melanoma according to tumor site
Corresponding author: Prof. Woo Jin Lee, Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea. uucm79@hanmail.net
-
Received: ,
Accepted: ,
How to cite this article: Jung JM, Jung CJ, Won CH, Chang SE, Lee MW, Choi JH, et al. Different progression pattern between acral and nonacral melanoma: A retrospective, comparative, clinicoprognostic study of 492 cases of primary cutaneous melanoma according to tumor site. Indian J Dermatol Venereol Leprol 2021;87:498-508.
Abstract
Background:
There are limited data regarding the difference in progression pattern between acral melanoma and nonacral melanoma.
Aims:
The objectives of this study were to compare the progression pattern between acral and nonacral melanoma and evaluate its impact on clinical outcomes.
Methods:
Clinical and histopathological features, survival outcomes and prognostic factors of 492 patients with acral melanoma or nonacral melanoma were retrospectively evaluated using the Asan Medical Center database.
Results:
The male-to-female ratio and the mean age was 1:0.92 and 60.2 years for acral melanoma (n = 249), and 1:0.85 and 58.4 years for nonacral melanoma (n = 243), respectively. The demographic difference was not significant. Although prediagnosis duration was longer and the advanced stage was more common in acral melanoma than that in nonacral melanoma, the vertical growth phase was more common in nonacral melanoma than that in acral melanoma, whereas, the horizontal diameter is longer in acral melanoma than that in nonacral melanoma. Dissemination to lymph nodes was more common in acral melanoma than that in nonacral melanoma. Lymph node involvement was associated with deeper Breslow thickness in nonacral melanoma but not in acral melanoma. The degree of correlation of prediagnosis duration with horizontal diameter was remarkable in acral melanoma, but with Breslow thickness in nonacral melanoma. Overall survival was worse in acral melanoma than that in nonacral melanoma. The prognostic value of Breslow thickness was more remarkable in nonacral melanoma than that in acral melanoma.
Limitations:
This study is a retrospective, single-center design.
Conclusion:
Acral melanoma has a longer radial growth phase compared with nonacral melanoma. However, acral melanoma is commonly associated with lymph node dissemination which contributed to worse survival in acral melanoma than nonacral melanoma.
Keywords
Melanoma
pathology
prognosis
survival
Plain Language Summary
Melanoma is one of the fatal skin cancers. In this study of 492 cases of melanoma, those arising from palms, soles and nails have a longer period of growing horizontally than melanoma from other areas. However, melanoma from palms, soles and nails are more prone to have lymph node involvement, which accounts for their poorer prognosis compared to other melanoma. Lymph node inspection is important when evaluating melanoma originating from palms, soles and nails.
Introduction
About half of all cutaneous melanomas in the Asian population and 60–70% in the black-skinned population are located in the acral area.1-4 Although acral melanoma has been widely evaluated, there are limited data available to analyze and compare clinicopathological features and spreading patterns between acral and nonacral melanoma. It has been unclear why acral melanoma has a worse prognosis, and this could be related to a delay of diagnosis or intrinsically, more aggressiveness behavior of the tumor.5-9 The mean Breslow thickness in acral melanoma has been known to be deeper than nonacral melanoma, but there has been conflicting data.7,10-14
Although acral lentiginous melanoma presents similar histological features with lentigo maligna melanoma, the biological behavior of acral lentiginous melanoma is more aggressive than that of lentigo maligna melanoma.1,15-17 However, specific populations afflicted by more indolent tumors with the long radial growth phase of acral melanoma have been reported.18 Huge acral melanoma with a clinical diameter measuring 4–6 cm without dermal invasion has been described.18,19 A peculiar spreading pattern has been observed in acral melanoma which may be associated with predisposing factors, anatomical structures and genetic predisposition that differ from non-acral melanoma.20,21
We performed this study to elucidate whether there is any difference in progression pattern between acral and nonacral melanoma. This study will be helpful in understanding different biological behaviors between acral and nonacral melanoma.
Methods
The institutional review board of Asan Medical Center, a tertiary referral center in South Korea, approved this study. We searched the Asan Medical Center database for cases of primary cutaneous melanoma that had been confirmed by skin biopsy between January 1996 and December 2017. Patients were grouped as acral melanoma (group A) and nonacral melanoma (group B). We defined acral melanoma as melanoma arising from the volar areas in hands and feet and nail apparatus. We excluded the patients whose prediagnosis duration and excisional specimens were not available.
Variables of interest
The following clinical data were collected: age at diagnosis, sex of patients, prediagnosis duration, the location of the lesion, lymph node invasion, and distant visceral metastasis at initial diagnosis. The prediagnosis duration was defined as the time interval between initial detection by patients and initial diagnosis.
The following data from the excisional biopsy slides were analyzed: histopathologic subtype, Breslow thickness, horizontal diameter, the presence of vertical growth phase, ulceration and mitotic activity. The horizontal diameter was defined as the longest distance between two points located on the lesion border.
The follow-up data, including date of recurrence and clinical status at the latest visit, were retrieved. Overall survival was calculated from the date of the initial diagnosis to the date of death from any cause or the last follow-up examination. Prognostic factors associated with survival were evaluated using clinical data at the time of initial staging. The stage of disease was based on the classification of the American Joint Committee on Cancer.22
Statistical analysis
Survival analysis was performed using the Kaplan–Meier method, and significance was tested using the log-rank test. Parameters affecting survival outcomes were assessed by univariate analysis. Prognostic factors at the time of diagnosis independently associated with overall survival were identified by multivariate analysis using Cox proportional hazards regression modeling. Comparisons between subgroups of patients were made using a Chi-square test or by a linear association test for categorical variables, and a t-test or Mann–Whitney test was conducted for continuous variables. All analyses were undertaken using Statistical Package for the Social Sciences (version 18.0; SPSS, Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
The retrospective review revealed 492 cases of primary cutaneous melanoma, of which 249 were cases of acral melanoma (group A) [Figures 1a-c], and 243 were cases of nonacral melanoma (group B) [Figures 1d-f].
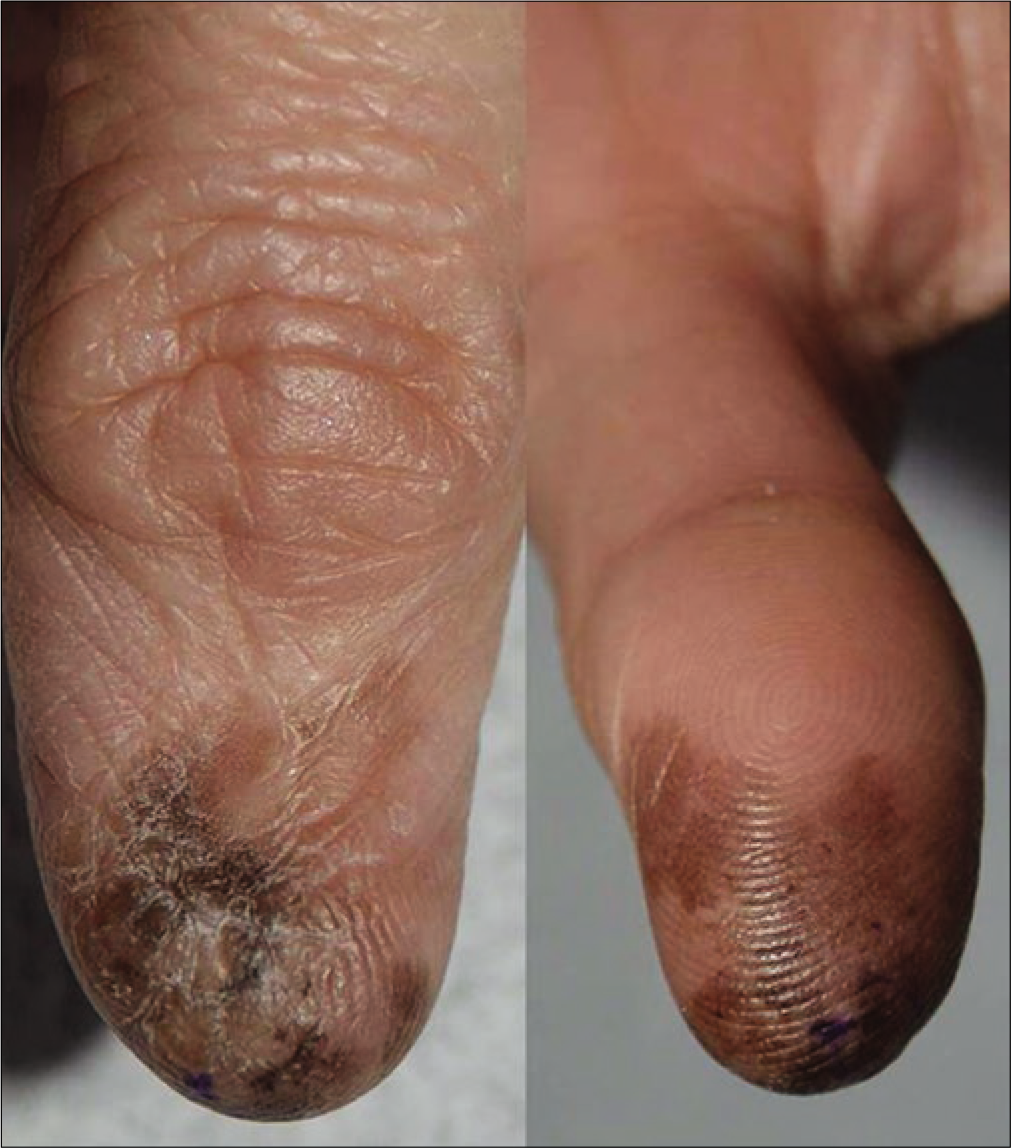
- Melanoma in situ with prediagnosis duration of 15 years in right thumb

- Melanoma in situ presenting with black colored patch with 6 cm diameter in left sole
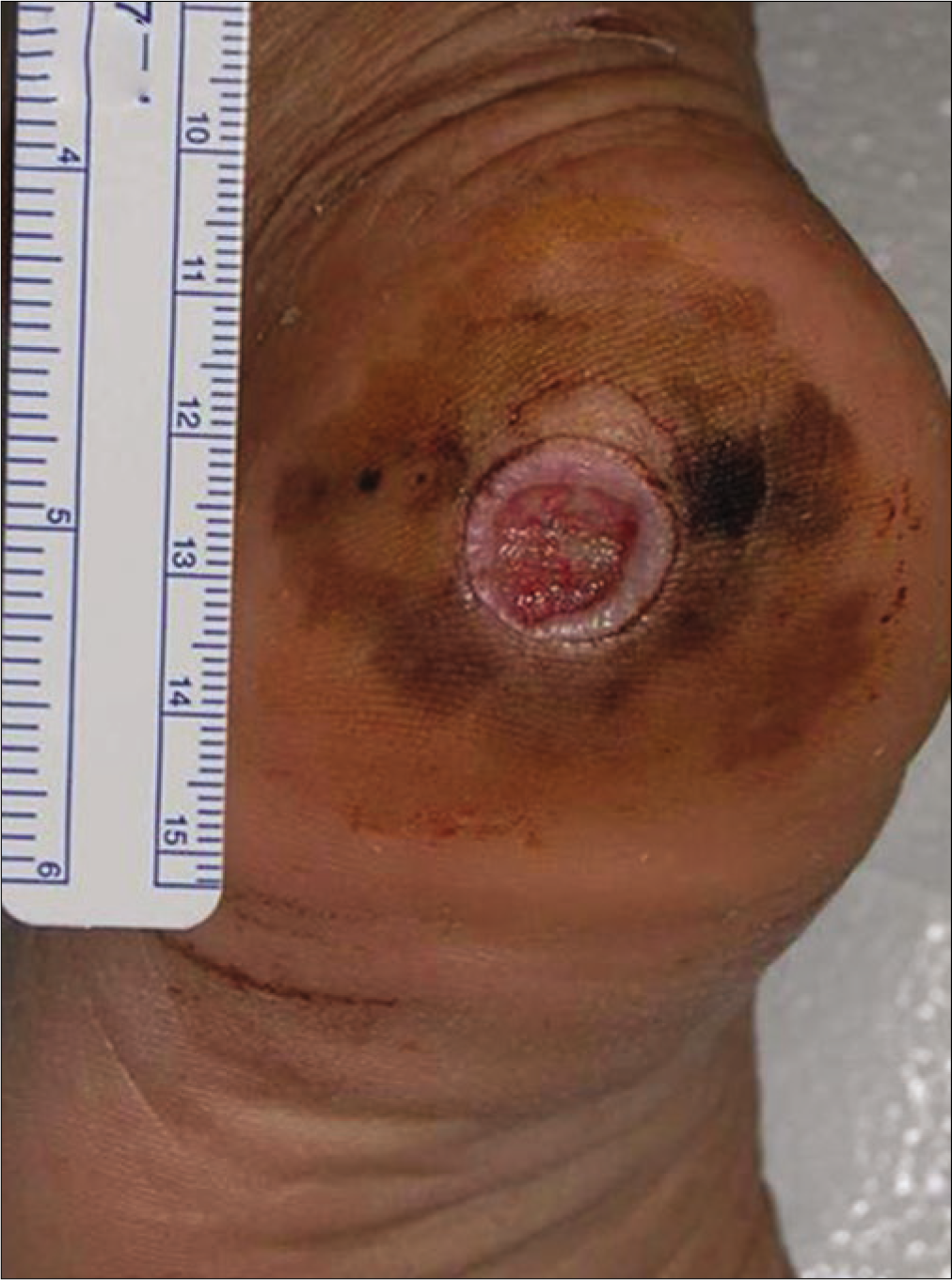
- Melanoma with T2 stage accompanying dissemination to inguinal lymph node
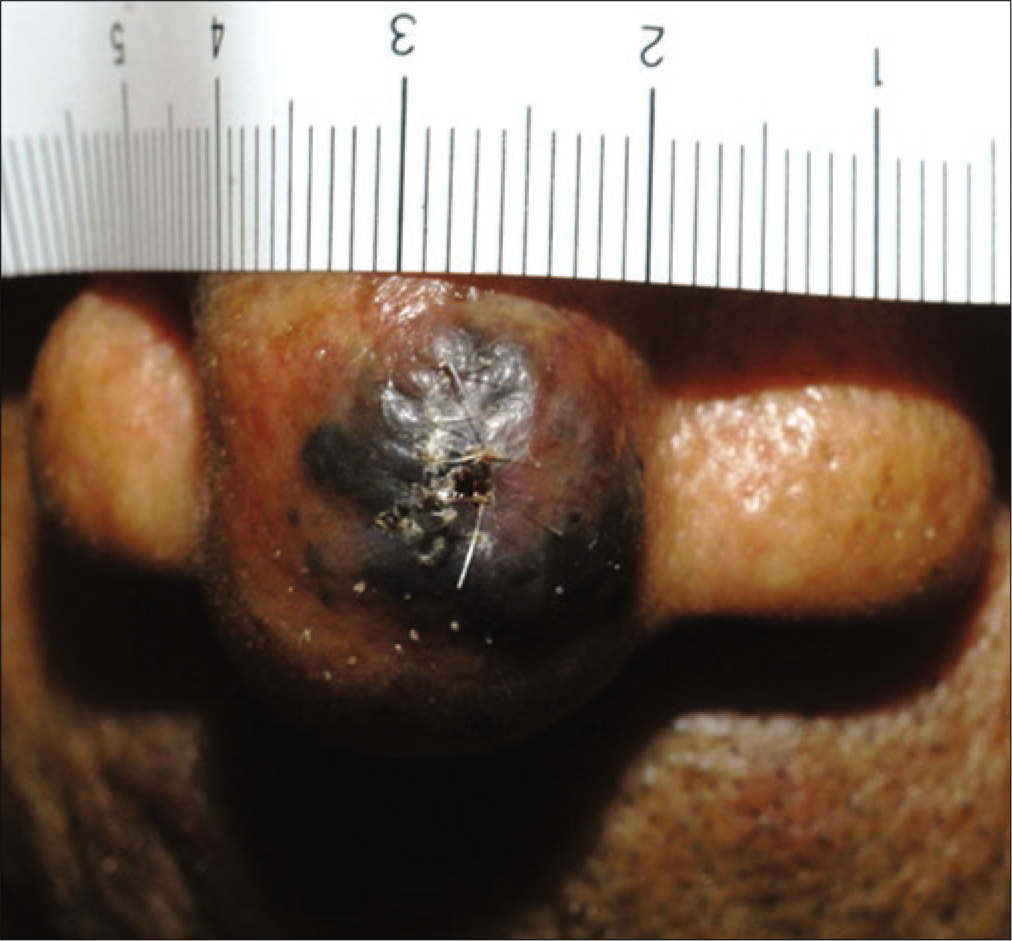
- Nodular melanoma with T3 stage on the nose
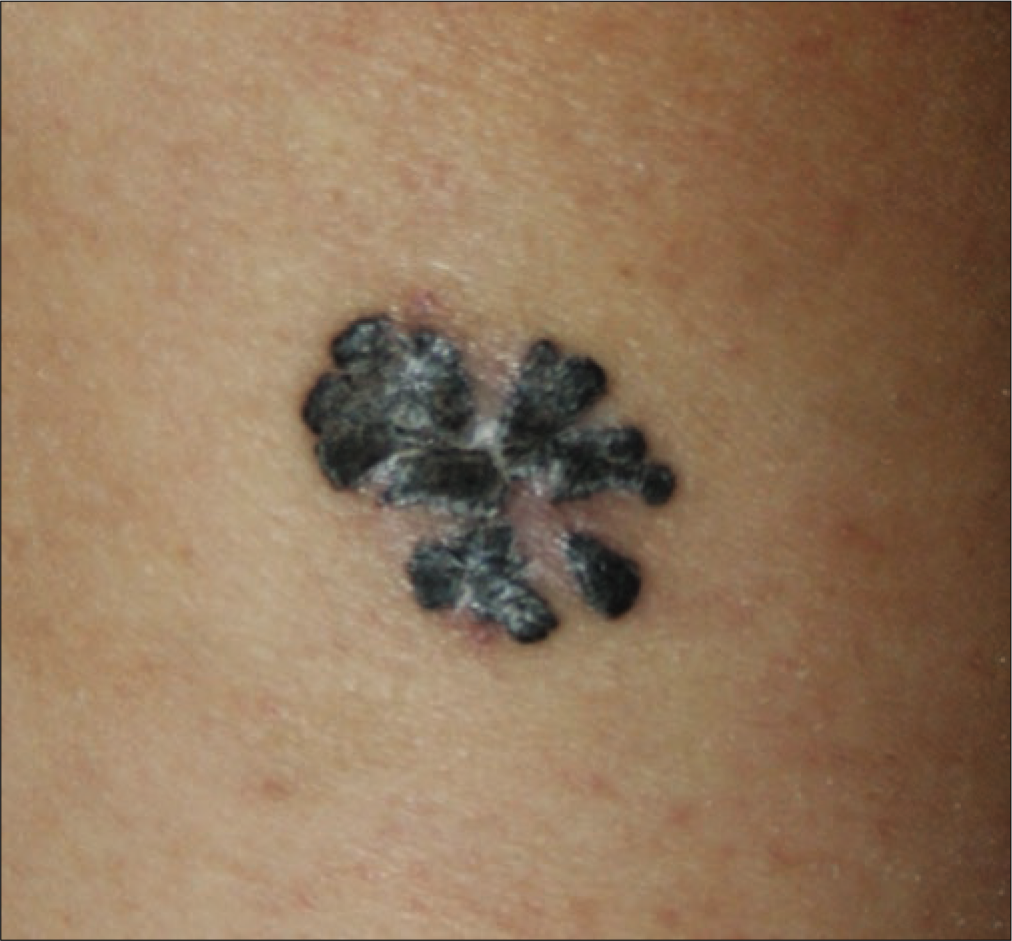
- Melanoma with T2 stage on left leg
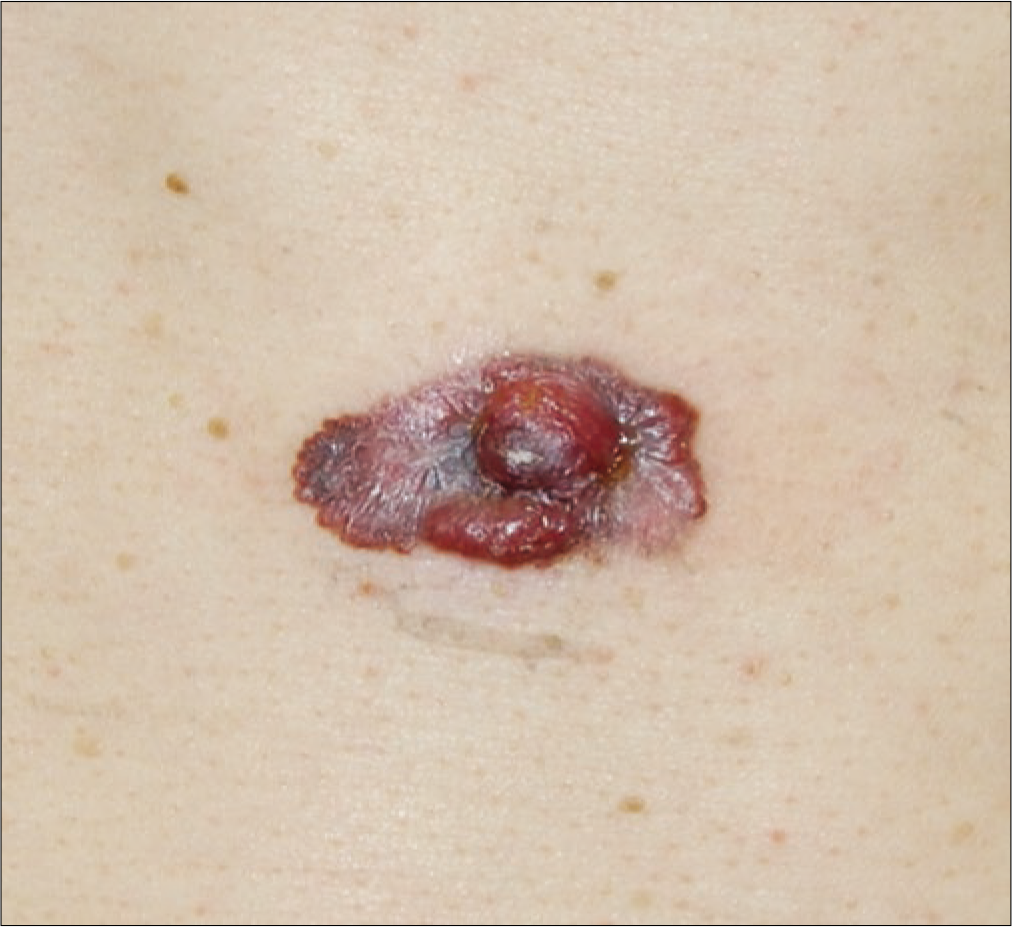
- Amelanotic nodular melanoma on the chest
Clinical features of acral melanoma and nonacral melanoma
The demographic data and clinical features of the patients with primary cutaneous melanoma are summarized in Table 1. The patients comprised 261 males and 231 females. There was no significant difference in the age of the patients between group A and group B (P = 0.517).
| Feature | Group A (n=249), n(%) | Group B (n=243), n(%) | P |
|---|---|---|---|
| Sex | |||
| Male | 129/249 (51.8) | 132/243 (54.3) | 0.576 |
| Female | 120/249 (48.2) | 111/243 (45.7) | |
| Age (years) | |||
| Range | 22-87 | 29-83 | 0.417 |
| Mean | 60.2 | 58.4 | |
| Prediagnosis duration | 44.63±18.37 | 22.28±11.10 | <0.001* |
| Site of lesion | |||
| Fingernail | 48/249 (19.3) | ||
| Toenail | 33/249 (13.3) | ||
| Palm | 8/249 (3.2) | ||
| Sole | 135/249 (54.2) | ||
| Finger | 7/249 (2.8) | ||
| Toe | 18/249 (7.2) | ||
| Head and neck | 59/243 (24.3) | ||
| Trunk | 69/243 (27.7) | ||
| Arms | 62/243 (25.5) | ||
| Legs | 53/243 (21.8) | ||
| Extracutaneous involvement at diagnosis | |||
| Lymph node involvement | 53/249 (21.3) | 34/243 (14.0) | 0.034* |
| Visceral involvement | 25/249 (10.0) | 13/243 (5.3) | 0.049* |
| AJCC stage at diagnosis | |||
| I/II | 192/249 (77.1) | 206/243 (84.8) | 0.031* |
| III/IV | 57/249 (22.9) | 37/243 (15.2) | |
| Breslow thickness, mm | 2.87±0.92 mm | 2.68±0.87 mm | 0.078 |
| ≤1 (T1) | 64/249 (25.7) | 49/243 (20.2) | |
| >1-≤2 (T2) | 63/249 (25.3) | 49/243 (20.2) | |
| >2-≤4 (T3) | 58/249 (23.3) | 78/243 (32.1) | |
| >4 (T4) | 64/249 (25.7) | 67/243 (27.6) | |
| Breslow thickness in each AJCC stage (mm) | |||
| Stage I | 1.28±0.21 | 0.95±0.18 | |
| Stage II | 2.32±0.26 | 3.09±0.33 | |
| Stage III | 2.65±0.37 | 4.22±0.46 | |
| Stage IV | 3.02±0.55 | 5.60±0.68 | |
| Vertical growth phase | |||
| Yes | 65/249 (26.1) | 91/243 (37.4) | 0.007* |
| No | 184/249 (73.9) | 152/243 (62.6) | |
| Horizontal diameter (cm) | 3.23±0.58 | 2.03±0.65 | <0.001* |
| Mitosis (mean), 10 HPF | 7.8 | 6.7 | 0.550 |
| Ulceration | |||
| Yes (n=123) | 72/249 (28.9) | 51/243 (21.0) | 0.042* |
| No (n=369) | 177/249 (71.1) | 192/243 (79.0) |
The prediagnosis duration was significantly longer in group A than that in group B (P = 0.001). The mean interval from the onset of skin lesion to initial diagnosis in group A was 44.63 months. The frequency of advanced stage at initial diagnosis was higher in group A than that in group B (P = 0.031), as too, were the frequencies of dissemination to lymph node (P = 0.034) and other visceral organs (P = 0.049).
Histopathological subtypes in acral melanoma and nonacral melanoma
The histopathological findings in our cohorts are summarized in Table 1. The majority of group A cases were a pathologic subtype of acral lentiginous melanoma (222/249; 89.2%), 26 (10.4%) were nodular melanoma, and one was superficial spreading melanoma (0.5%). In group B, superficial spreading melanoma (90/243, 37.0%) was the most common pathologic subtype, followed by nodular melanoma (79/243, 32.5%), lentigo maligna melanoma (47/243, 19.3%) and acral lentiginous melanoma (27/243, 11.1%). Ulceration was significantly more common in group A than in group B (P = 0.042).
Breslow thickness and clinical features
The average recorded Breslow thickness was 2.69 mm (range 0.0–11.3 mm). Although the prediagnosis duration was longer, and advanced stage was more common in group A than that in group B, Breslow thickness was not significantly different between group A (2.87 ± 0.92 mm) and group B (2.68 ± 0.87 mm) (P = 0.078). However, the vertical growth phase was significantly more common in group B (91/243, 37.4%) than in group A (65/249, 26.1%, P = 0.007). When the correlations between Breslow thickness and the frequency of tumor dissemination to lymph node or viscera were evaluated, a significant correlation was found in group B (lymph node, P < 0.001; viscera, P = 0.007) but not in group A (lymph node, P = 1; viscera, P = 0.209). As Breslow’s thickness was getting deeper, the frequency of lymph node dissemination was getting higher in group B but not in group A [Figure 2a]. The frequency of lymph node involvement was not different between the T2 and T3 stages in group A (P = 0.495). The frequency of stage III or IV was also associated with deeper Breslow thickness in group B (P < 0.001) but not in group A (P = 0.227) [Figure 2b]. There was a significant difference in the mean Breslow thickness between patients with stage I/II and stage III/IV in group B (P < 0.001), but not in group A (P = 0.791) [Figure 2c]. In T-stage matched comparison, the frequency of lymph node dissemination was higher in group A compared with group B in the T1 (P = 0.01), T2 (P = 0.04) and T3 (P = 0.017) stages but not in the T4 (P = 0.311) stage.
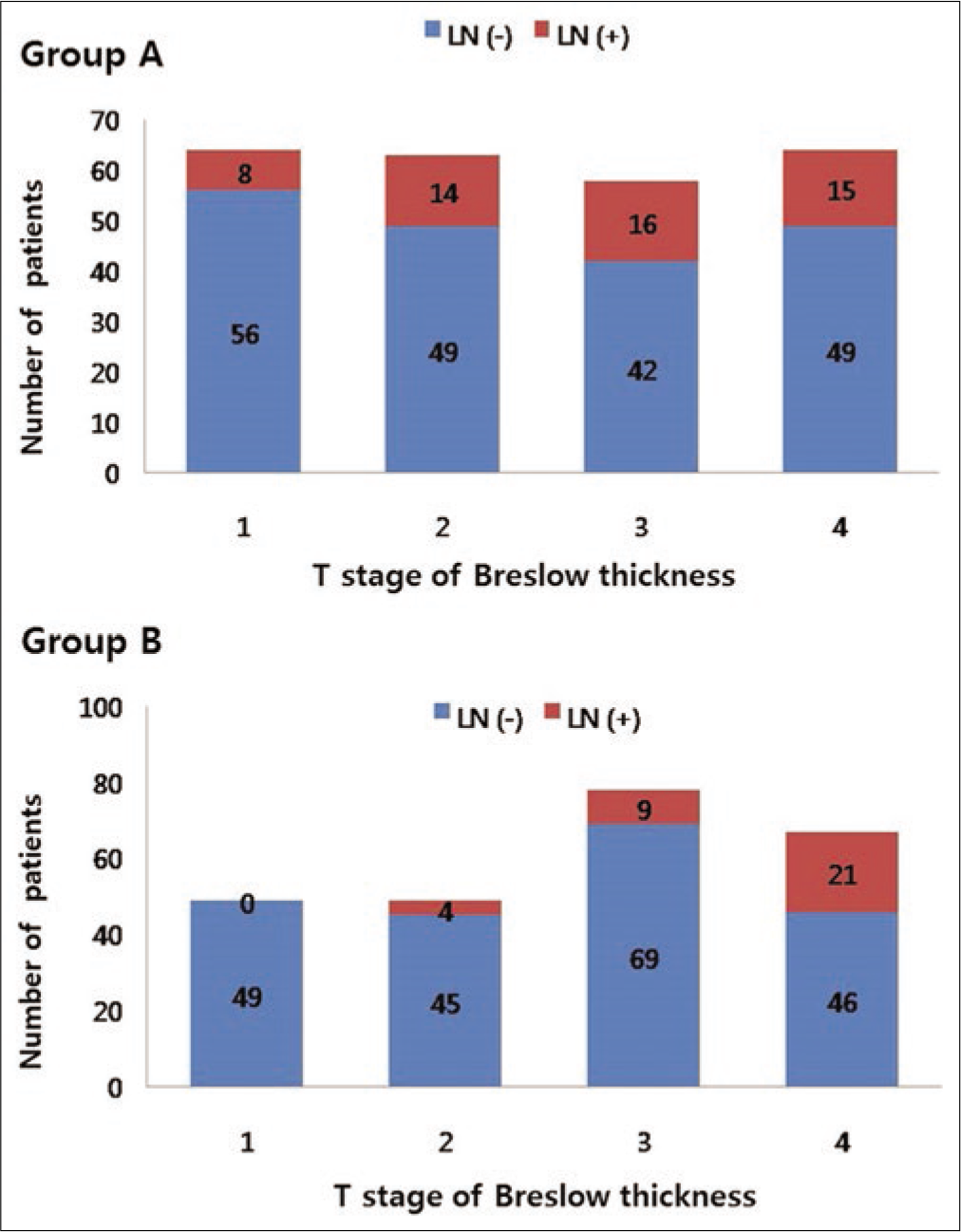
- The significance of Breslow thickness and prediagnosis duration. The differences in the frequency of lymph node dissemination depending on Breslow thickness
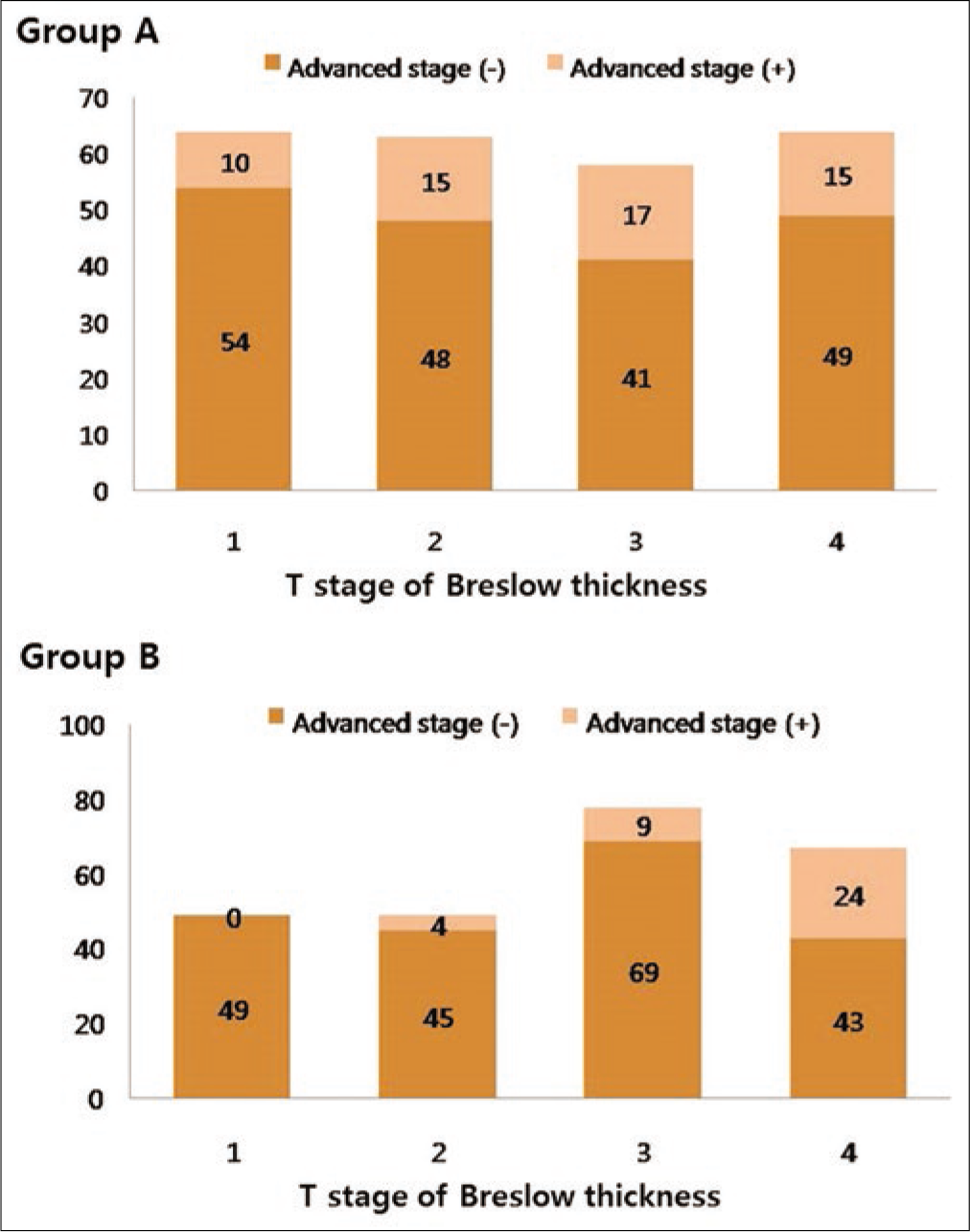
- The significance of Breslow thickness and prediagnosis duration. The differences in the frequency of advanced American Joint Committee on Cancer stage depending on Breslow thickness
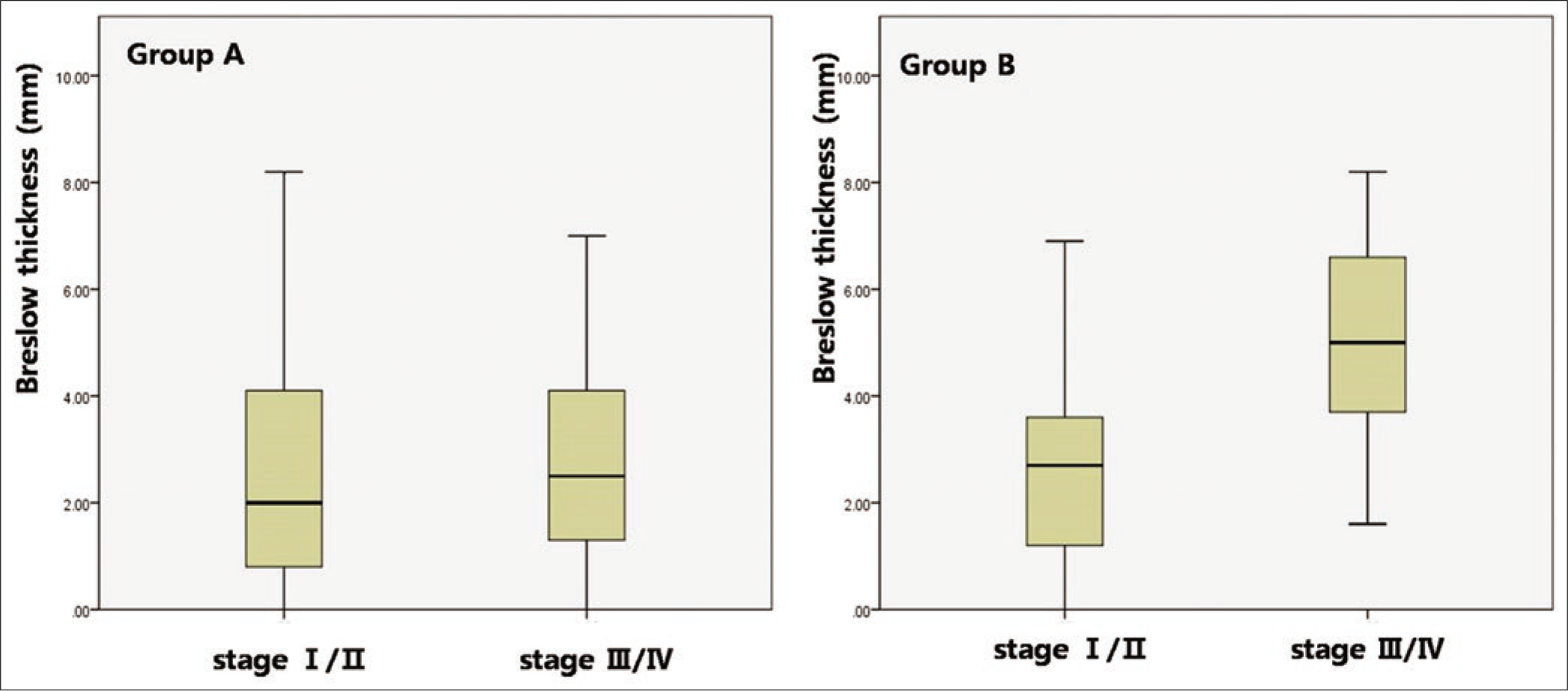
- The significance of Breslow thickness and prediagnosis duration. Comparison of mean Breslow thickness between patients with Stage I/II and Stage III/IV in Groups A and B
Horizontal diameter in acral melanoma and nonacral melanoma
The horizontal diameter was significantly longer in group A (3.23 ± 0.58 cm) than that in group B (2.03 ± 0.65 cm) (P < 0.001). After longer prediagnosis duration in group A was adjusted, the horizontal diameter remained longer in group A than that in group B (P < 0.001). The horizontal diameter was not significantly different, depending on lymph node dissemination and advanced stage, in both group A (lymph node, P = 0.227; stage, P = 0.349) and group B (lymph node, P = 0.322; stage, P = 0.216).
Prediagnosis duration and clinicopathological features
There was no significant difference in prediagnosis duration of patients with lymph node dissemination at initial diagnosis between group A (38.21 ± 10.28) and group B (34.41 ± 9.23, P = 0.062). However, lymph node dissemination at initial diagnosis in group B was mainly found among patients with long prediagnosis duration, but this tendency was not found in group A [Figure 2d]. The prediagnosis duration in patients with vertical growth phase was significantly longer in group A (47.98 ± 15.21) than that in group B (32.42 ± 9.04, P < 0.001). The degree of correlation between horizontal diameter and prediagnosis duration was more remarkable in group A (k = 0.739, P < 0.001) than that in group B (k = 0.451, P < 0.001) [Figure 2e], whereas, the degree of correlation between Breslow thickness and prediagnosis duration was more remarkable in group B (k = 0.887, P < 0.001) than that in group A (k = 0.422, P < 0.001) [Figure 2f].
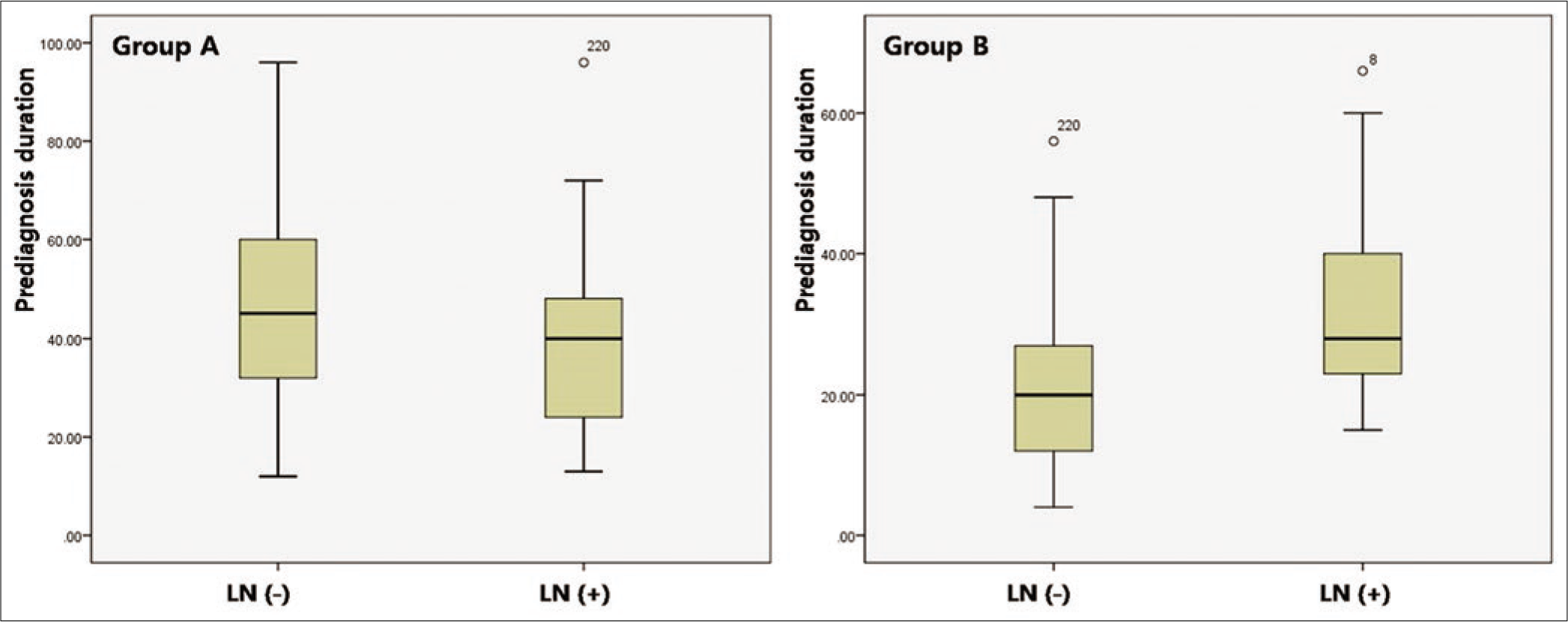
- The significance of Breslow thickness and prediagnosis duration. The difference in prediagnosis duration depending on lymph node status at initial diagnosis
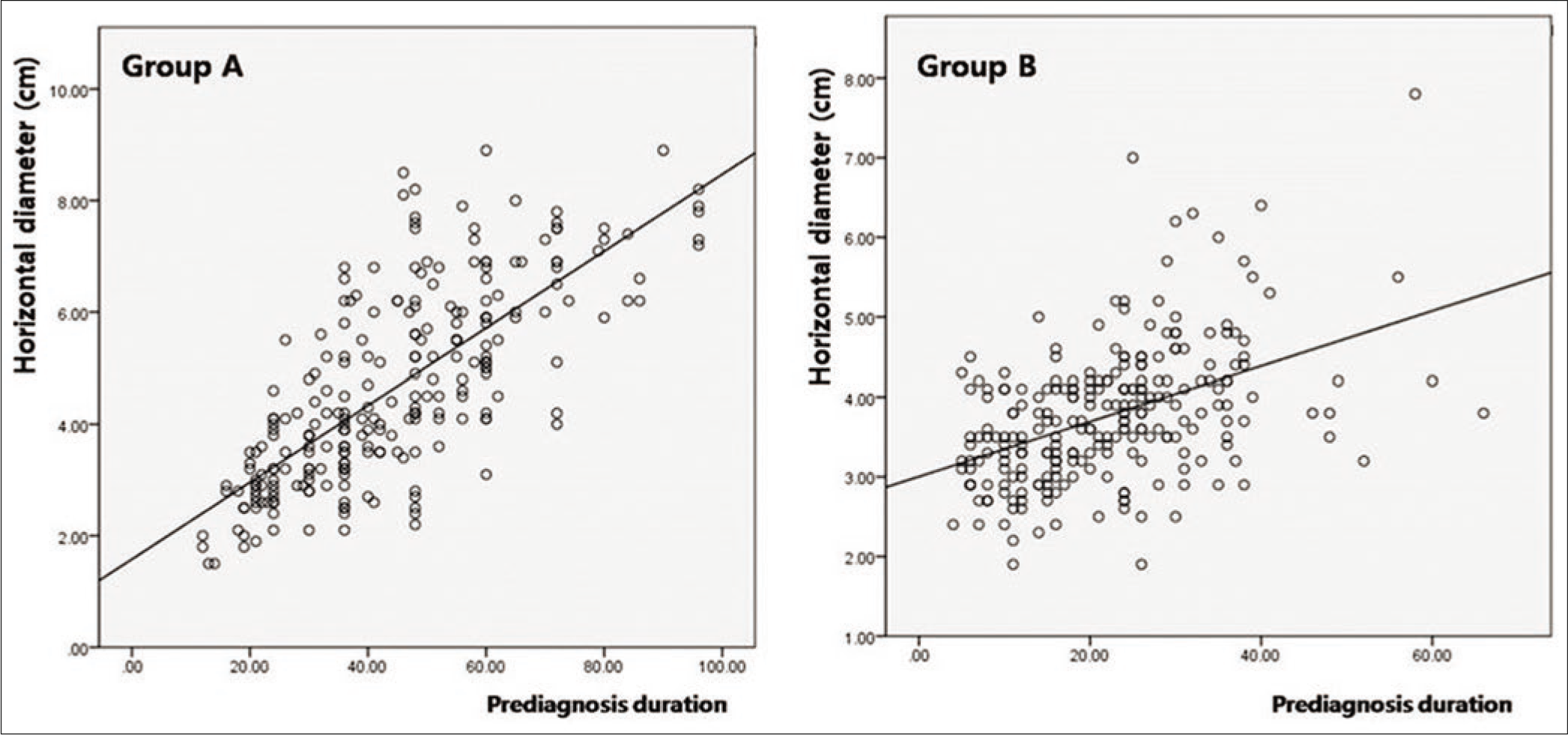
- The significance of Breslow thickness and prediagnosis duration. The correlation between lesional diameter and prediagnosis duration
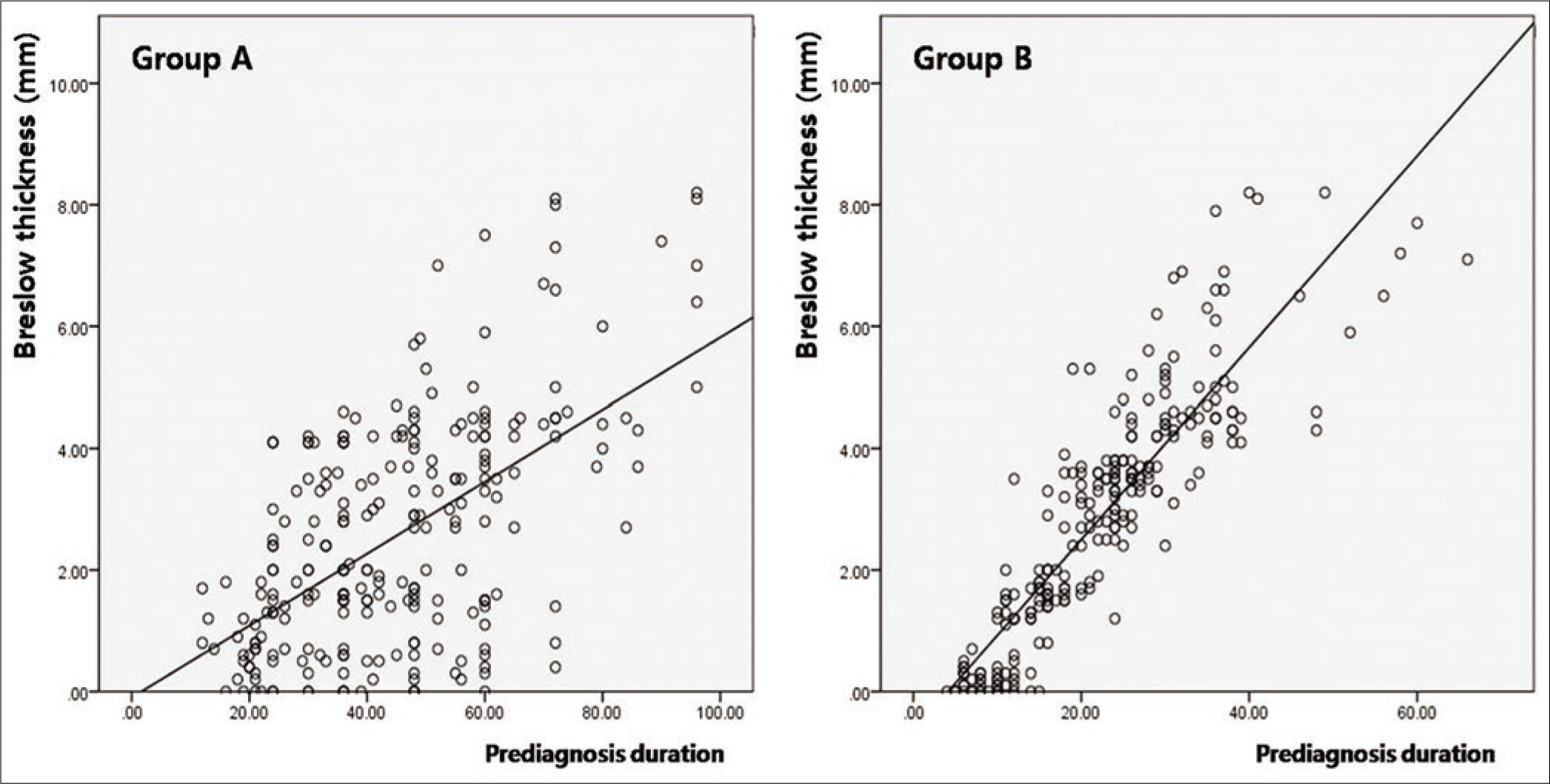
- The significance of Breslow thickness and prediagnosis duration. The correlation between Breslow thickness and prediagnosis duration
Survival outcomes
The five-year overall survival rate of the whole cohort was 64%. Both overall survival (P = 0.011) and progression-free survival (P = 0.009) were significantly worse in group A relative to group B [Figure 3a]. When overall survival was compared between T-staged matched patients, it was worse in group A than that in group B in T1 (P < 0.001) [Figure 3b], T2 (P = 0.026) [Figure 3c] and T3 (P = 0.009) [Figure 3d] stages. However, there was no significant difference in T4 patients between group A and group B (P = 0.846) [Figure 3e].
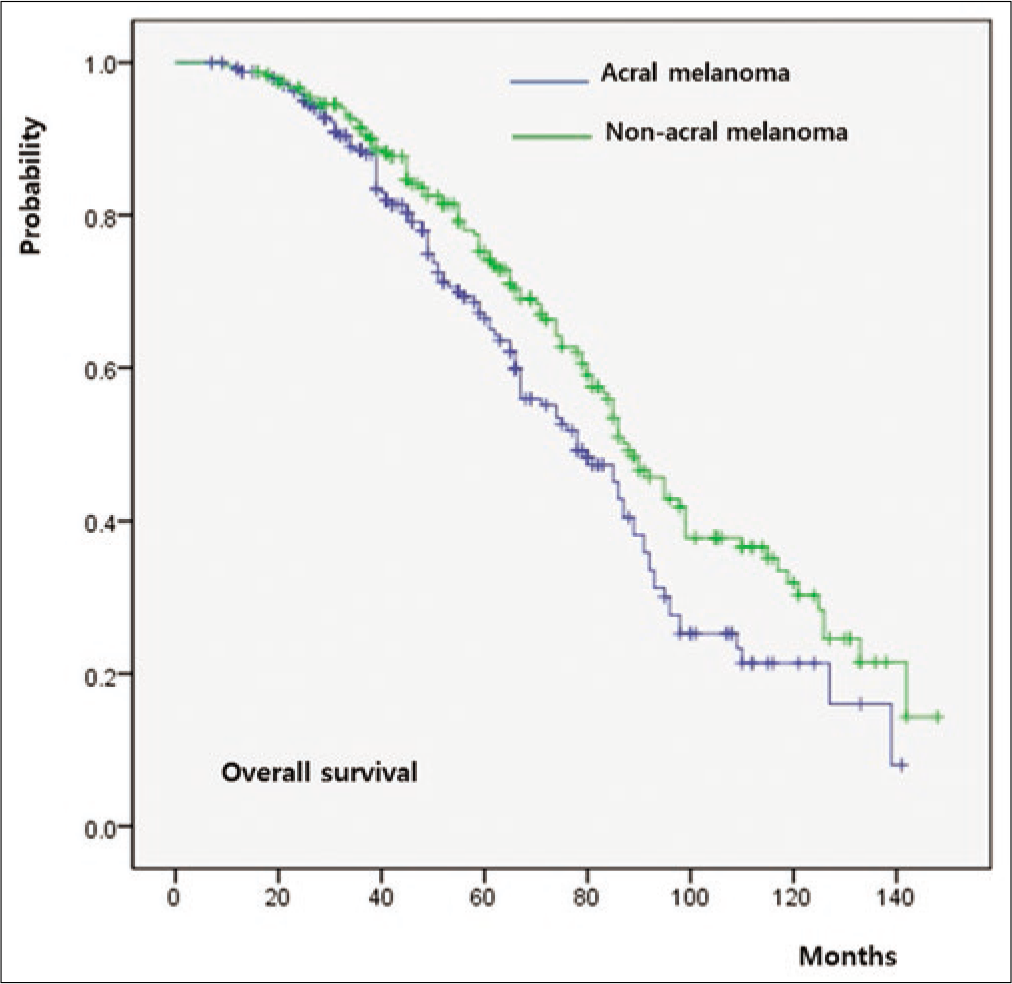
- Survival outcomes in acral melanoma and nonacral melanoma. Comparison of overall survival between Group A and Group B. T-staged matched comparison of overall survival in patients with
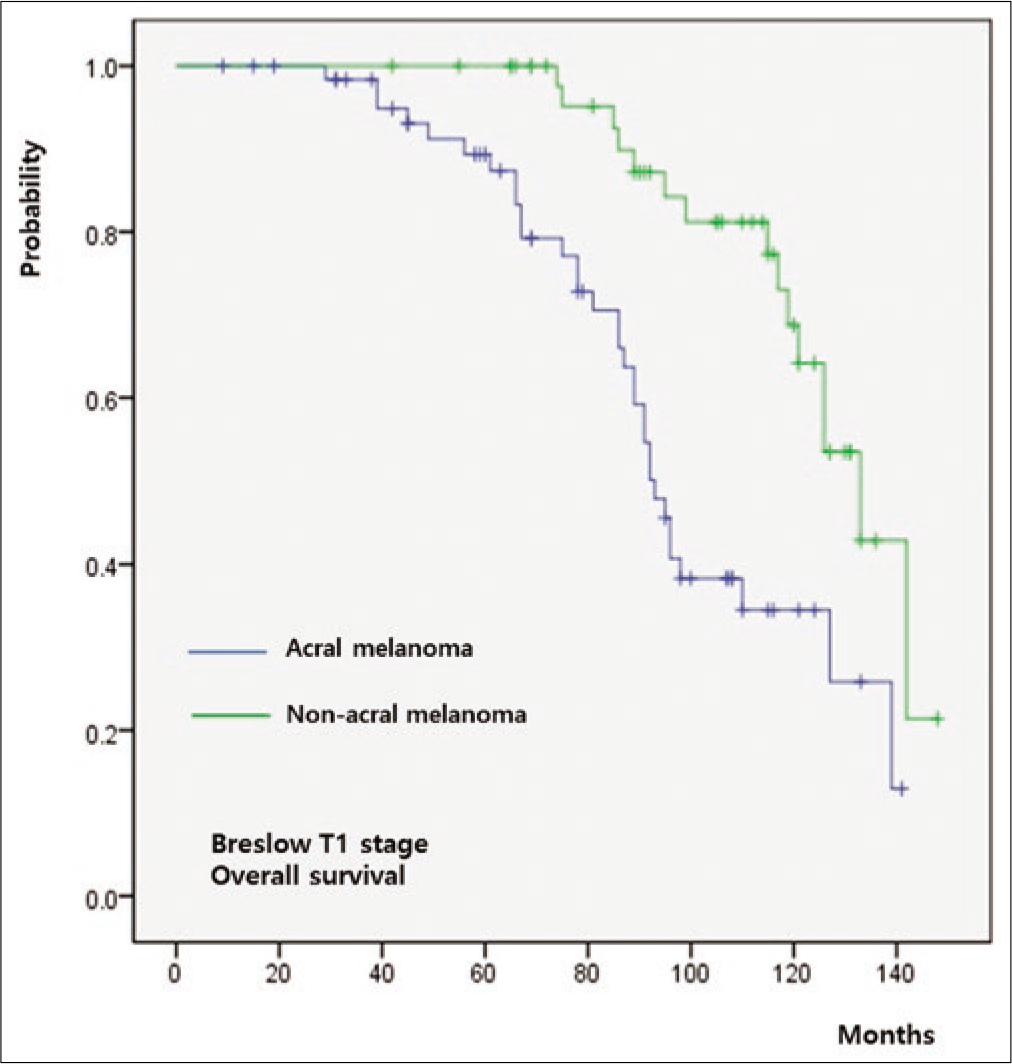
- Survival outcomes in acral melanoma and nonacral melanoma. T1 stage
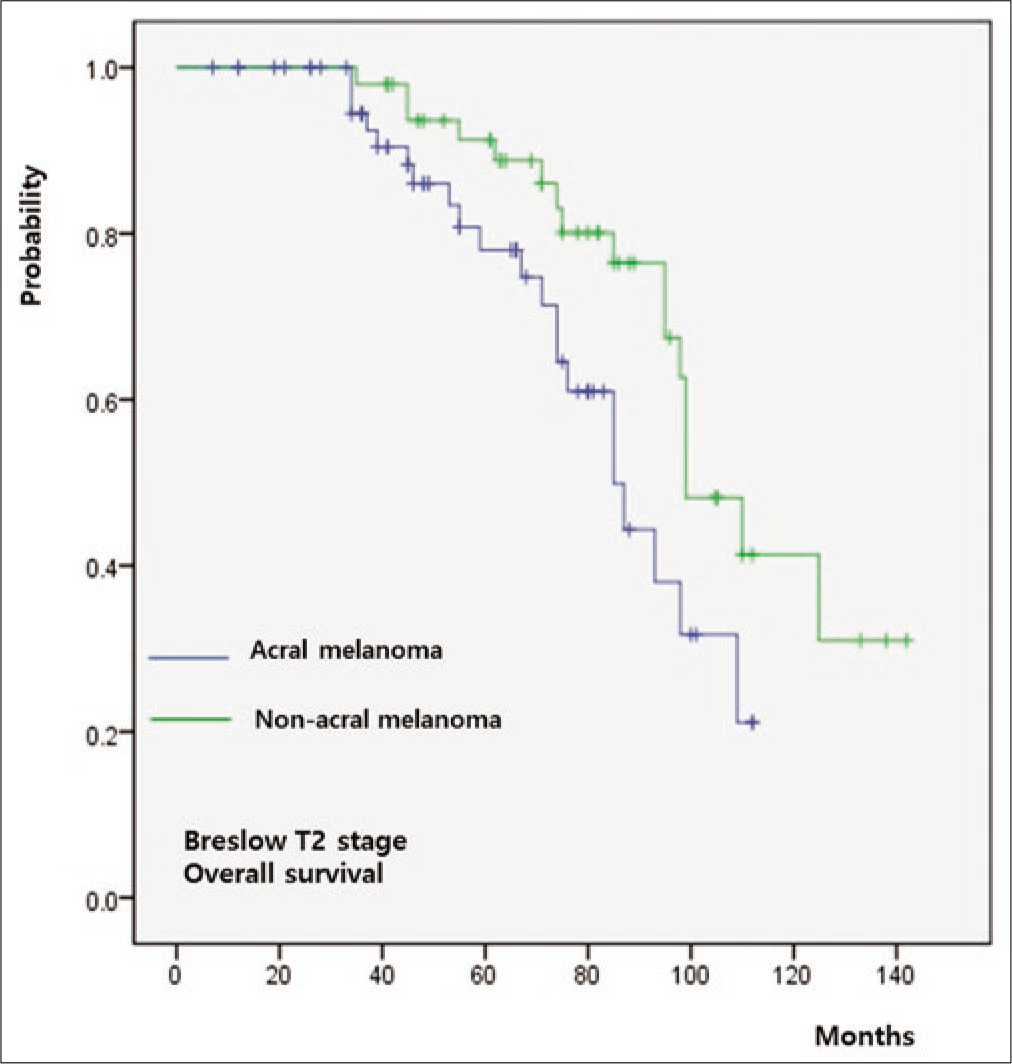
- Survival outcomes in acral melanoma and nonacral melanoma. T2 stage
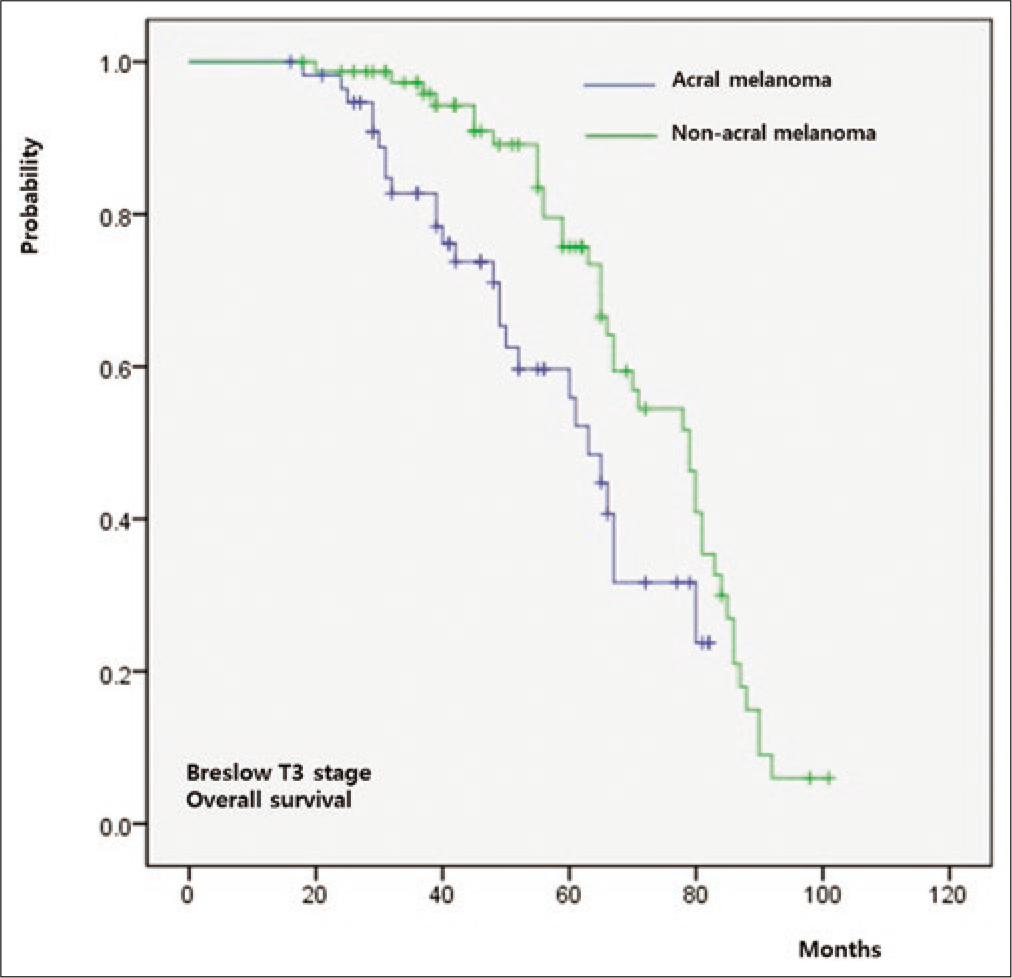
- Survival outcomes in acral melanoma and nonacral melanoma. T3 stage
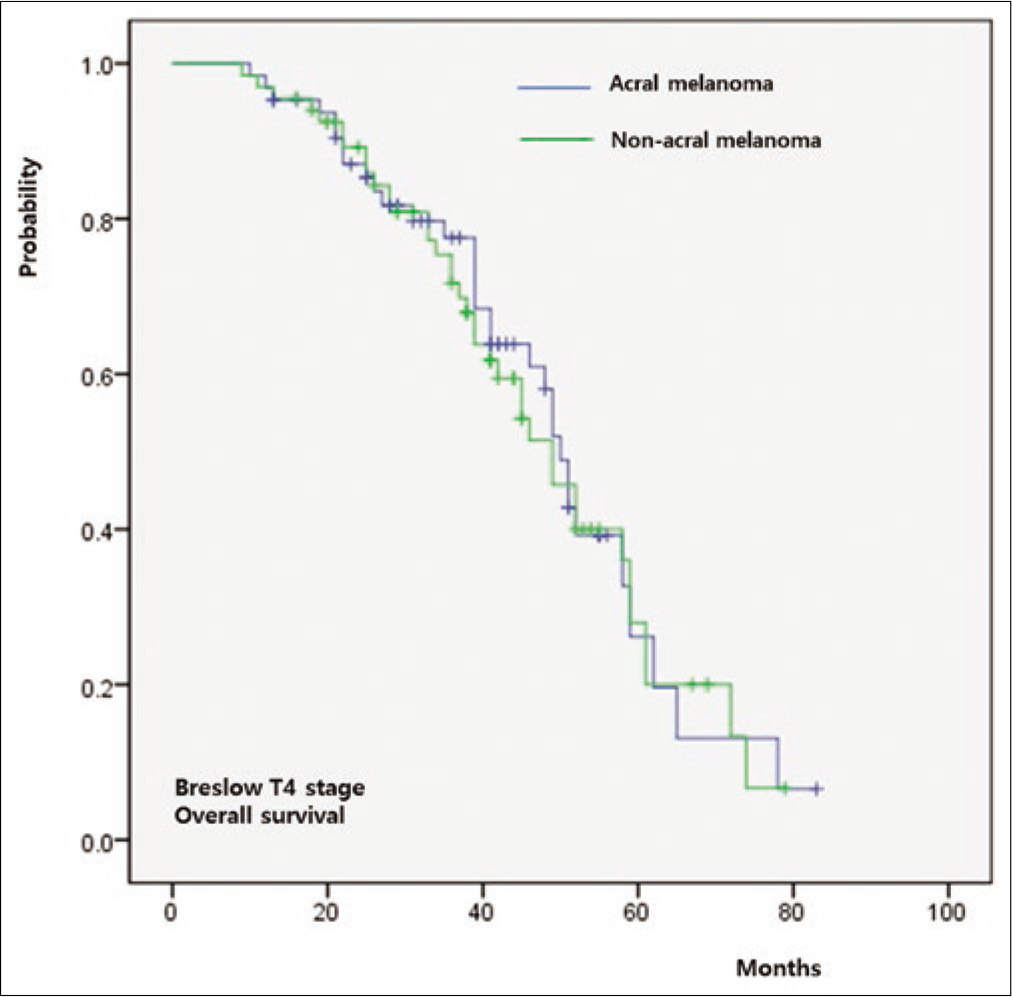
- Survival outcomes in acral melanoma and nonacral melanoma. T4 stage
Univariate and multivariate analyses are summarized in Table 2. Multivariate analysis showed that advanced stage, the involvement of lymph node and Breslow thickness were associated independently with a poorer prognosis in both group A and group B. The hazard ratio for Breslow thickness was higher in group B (hazard ratio, 2.91; 95% confidence interval 1.38–4.88;P = 0.019) than group A (hazard ratio, 2.32; 95% confidence interval 1.33–4.69;P = 0.0028).
| Covariate | Group A | Group B | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariate analysis | ||||||
| Age (years) | ||||||
| <60 versus ≥60 | 0.93 | 0.62-2.88 | 0.328 | 0.98 | 0.68-2.92 | 0.412 |
| Sex | ||||||
| Female versus male | 1.79 | 0.65-3.28 | 0.414 | 1.82 | 0.79-4.12 | 0.492 |
| AJCC stage | ||||||
| Early versus advanced | 3.12 | 1.24-4.54 | <0.001* | 3.08 | 1.34-4.53 | <0.001* |
| Involvement of LN | ||||||
| Yes versus no | 2.41 | 1.10-4.78 | 0.008* | 1.89 | 1.16-5.74 | 0.021* |
| Breslow thickness | ||||||
| T1 or T2 versus T3 or T4 | 2.32 | 1.33-4.69 | 0.028* | 2.91 | 1.38-4.88 | 0.019* |
| Lymphovascular invasion | ||||||
| Yes versus no | 1.01 | 0.87-5.61 | 0.471 | 0.95 | 0.79-5.02 | 0.366 |
| Vertical growth phase | ||||||
| Yes versus no | 2.29 | 1.11-5.10 | 0.012* | 2.02 | 1.13-4.83 | 0.028* |
| Ulceration | ||||||
| Yes versus no | 1.21 | 0.92-5.33 | 0.573 | 1.18 | 0.85-5.11 | 0.332 |
| Multivariate analysis | ||||||
| AJCC stage | ||||||
| Early versus advanced | 2.28 | 1.21-4.59 | 0.021* | 2.31 | 1.23-4.44 | 0.032* |
| Involvement of LN | ||||||
| Yes versus no | 2.21 | 1.18-4.81 | 0.022* | 1.55 | 1.14-4.77 | 0.039* |
| Breslow thickness | ||||||
| T1 or T2 versus T3 or T4 | 1.22 | 1.21-5.01 | 0.039* | 1.97 | 1.12-4.85 | 0.031* |
| Vertical growth phase | ||||||
| Yes versus no | 1.62 | 1.09-4.74 | 0.039* | 1.66 | 1.18-4.75 | 0.042* |
*Statistically significant. Group A: Acral melanoma, Group B: Nonacral melanoma, LN: Lymph node, AJCC: American Joint Committee on Cancer, CI: Confidence interval, HR: Hazard ratio
Discussion
Recent studies have demonstrated that acral melanoma has poorer survival outcomes than nonacral melanoma, even after controlling for American Joint Committee on Cancer stage,13,23 suggesting poor outcomes of acral melanoma are related to inherent biological differences.24-27
In contrast to nonacral melanoma, long-term physical stress is an important causative factor of acral melanoma.4,20,28,29 Acral melanoma is associated with KIT mutation and has a lower incidence of the BRAF or NRAS mutation which are frequently detected in other subtypes of melanoma.30,31 One previous study suggested that unique features of acral melanoma are associated with constitutive activation of the phosphatidylinositol 3-kinase pathway.32 Acral melanoma is known to demonstrate a high frequency of focal amplifications (such as CCND1 and CDK4) and low mutational rates compared to nonacral melanoma.24,33 These genetic differences would result in peculiar clinicopathologic features and different biological behavior of acral melanoma from other subtypes. Since differences in pathogenesis contributing to worse survival in acral melanoma have not been fully elucidated, we performed this study to evaluate whether there is a difference in progression pattern between acral and nonacral melanoma.
In this study, Breslow’s thickness was not significantly different between acral and nonacral melanoma. Many previous studies have shown that acral melanoma has deeper Breslow thickness than nonacral melanoma. When compared to the western population,34,35 superficial spreading melanoma is relatively rare and nodular melanoma is relatively common among nonacral melanoma in our melanoma cohort. In this study, superficial spreading melanoma comprised 37% of nonacral melanoma and is relatively uncommon considering previous data from western populations.34,35 Moreover, indolent acral melanoma with long radial growth phase has been reported in Asian populations, and we found many similar cases of acral melanoma presenting longer horizontal diameter than tumor thickness in our cohort.18,19 These observations may contribute to explaining the lack of significant difference in Breslow thickness between acral and nonacral melanoma in this study.
Similar to previous studies, prediagnosis duration was longer in acral melanoma than that in nonacral melanoma. Phan et al. reported that the lesion duration is longer than 2 years in 31% of acral melanoma.4 The delay between the first notice by the patient and the diagnosis of acral melanoma ranged from 2 to 204 months, in our study. Although there was no significant difference in Breslow thickness, the horizontal diameter is significantly longer in acral melanoma even after correcting for the longer prediagnosis duration. Although prediagnosis duration was significantly longer in acral melanoma, the vertical growth phase was less common in acral melanoma compared with nonacral melanoma. In acral melanoma, the horizontal diameter was well correlated with prediagnosis duration, but Breslow thickness was remarkably correlated with prediagnosis duration in nonacral melanoma, indicating differences in the spreading pattern between acral and nonacral melanoma, and a longer radial growth phase in acral melanoma. Several factors may contribute to the tendency of longer radial growth in acral melanoma. First, acral melanoma occurs on more physically stressed sites with the long axis along natural creases on the sole.20 Naturally occurring creases in the volar areas could be related to the tendency of radial growth rather than the vertical growth. The palmoplantar area has relatively firm soft tissue, resulting in resistance to dermal invasion. In nail unit melanoma, the extent of invasion could be variable in spots of the nail unit, both lengthwise and breadthwise.36 Shin et al. revealed that the nail matrix is highly resistant to vertical invasion, due to the presence of the onychodermis.21
The 5-year survival rate of acral melanoma in this study was 64%, which is similar to other Asian series.37,38 The survival of patients with acral melanoma stratifies along with increasing American Joint Committee on Cancer stage. Moreover, this study evaluated acral melanoma by a T-stage matched comparison with nonacral melanoma. The overall survival of acral melanoma was poorer than that of nonacral melanoma in T-stage 1/2/3 when T-stage was matched. These matched outcomes are consistent with the Surveillance, Epidemiology and End Results population-based analysis.23 Worse prognosis in acral melanoma compared to nonacral melanoma would be related to frequent lymph node involvement at diagnosis in acral melanoma. Differences in the frequency of advanced American Joint Committee on Cancer stage between acral and nonacral melanoma at initial diagnosis could also be explained by lymph node status at diagnosis. It has been proven that 20 to 30% of patients with acral melanoma show positivity in sentinel lymph node biopsy.39,40 However, there was no significant difference in overall survival between T-stage 4 acral and nonacral melanoma patients. The frequency of lymph node involvement was significantly higher in acral melanoma than that in nonacral melanoma among T-stage 1, 2, and 3 patients, but not among T-stage 4 patients. In this study, the prognostic value of Breslow thickness was more remarkable in nonacral melanoma than that in acral melanoma. Since Breslow thickness was not related to lymph node involvement in acral melanoma, the prognostic value would be weaker in acral melanoma than that in nonacral melanoma.
Although prediagnosis duration was significantly longer in acral melanoma compared with nonacral melanoma, there were no significant differences in the prediagnosis duration of patients with lymph node dissemination at initial diagnosis between acral and nonacral melanoma. Significantly longer prediagnosis duration was associated with lymph node involvement in group B, but not in group A. It implies that lymph node involvement appears relatively earlier in acral melanoma than that in nonacral melanoma, during tumor progression. Lymph node involvement is significantly associated with deeper Breslow thickness in nonacral melanoma, but not in acral melanoma, indicating that lymph node dissemination is related to the vertical growth phase in nonacral melanoma but not in acral melanoma. Lymph node involvement is not rare even in acral melanoma with Breslow thickness T1 or T2. Since lymph node dissemination is not associated with vertical growth in the development ofacral melanoma, there was no significant difference in Breslow thickness between initial and advanced American Joint Committee on Cancer stage in acral melanoma.
The progression pattern in acral melanoma could be characterized by a longer radial growth phase and more tendency to develop lymph node dissemination compared to nonacral melanoma. The propensity of acral melanoma to recur locally and develop in-transit metastasis has been described previously.4,27,28 High frequency of locoregional recurrence in acral melanoma could not only be attributed to inadequate surgical margin due to complex anatomy but also related to the tendency of lymphatic dissemination in acral melanoma.
In summary, poor prognosis of acral melanoma could not only be contributed by the delayed diagnosis but also by an intrinsic aggressive spreading pattern. Although acral melanoma has a longer radial growth phase, lymph node dissemination is the main spreading pattern in acral melanoma. Lymph node surveillance is important in the follow-up and assessment of acral melanoma. Further studies including molecular analysis would help understand these differences in progression pattern between acral and nonacral melanoma.
Limitations
The limitation of this study is clinical data, such as prediagnosis duration could be based on uncertain information, because of its retrospective nature.
Conclusion
Acral melanoma has a longer radial growth phase compared with non-acral melanoma. However, acral melanoma is more prone to have lymph node dissemination, which accounts for its poorer prognosis compared to non-acral melanoma. Lymph node surveillance is important when evaluating acral melanoma.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by a National Research Foundation of Korea grant funded by the Korea government (MSIT) (NRF-2018R1C1B6003389).
Conflicts of interest
There are no conflicts of interest.
References
- Acral lentiginous melanoma. A clinicopathologic study of 36 patients. Cancer. 1983;52:161-8.
- [CrossRef] [Google Scholar]
- Incidence of cutaneous melanoma among non hispanic whites, hispanics, asians, and blacks: An analysis of california cancer registry data, 1988 93. Cancer Causes Control. 1997;8:246-52.
- [Google Scholar]
- Malignant melanoma in Taiwan: A prognostic study of 181 cases. Melanoma Res. 2004;14:537-41.
- [CrossRef] [PubMed] [Google Scholar]
- Acral lentiginous melanoma: A clinicoprognostic study of 126 cases. Br J Dermatol. 2006;155:561-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acral cutaneous melanoma in caucasians: Clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143:275-80.
- [CrossRef] [PubMed] [Google Scholar]
- Extent and consequences of physician delay in the diagnosis of acral melanoma. Melanoma Res. 1998;8:181-6.
- [CrossRef] [PubMed] [Google Scholar]
- Subungual malignant melanoma: Clinicopathological features of 100 cases. Histopathol. 1991;19:425-9.
- [CrossRef] [PubMed] [Google Scholar]
- Subungual melanoma: A clinico pathological study of 24 cases. Br J Plast Surg. 1992;45:275-8.
- [CrossRef] [Google Scholar]
- The Scottish melanoma group: A progress report, 159th meeting of the pathological society of Great Britain and Ireland. Aberdeen J Pathol. 1989;158:A335.
- [CrossRef] [PubMed] [Google Scholar]
- Subungual melanoma: A study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-12.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and prognosis of nail apparatus melanoma. A retrospective study of 105 patients in four English regions. Br J Dermatol. 1998;139:276-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acral lentiginous melanoma: Histopathological prognostic features of 121 cases. Br J Dermatol. 2007;157:311-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis of acral melanoma: A series of 281 patients. Ann Surg Oncol. 2013;20:3618-25.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological significance of tumor infiltrating lymphocytes and programmed death 1 expression in cutaneous melanoma: A comparative study on clinical subtypes. Melanoma Res. 2018;28:423-34.
- [CrossRef] [PubMed] [Google Scholar]
- Plantar lentiginous melanoma: A distinctive variant of human cutaneous malignant melanoma. Am J Surg Pathol. 1977;1:131-43.
- [CrossRef] [PubMed] [Google Scholar]
- Melanomas of the palm, sole, and nailbed: A clinicopathologic study. Cancer. 1980;46:2492-504.
- [CrossRef] [Google Scholar]
- Pigmented freckles on the sole of acral lentiginous melanoma in situ. J Dermatol. 1985;12:263-6.
- [CrossRef] [PubMed] [Google Scholar]
- Acral lentiginous melanoma: Indolent subtype with long radial growth phase. Am J Dermatopathol. 2014;36:142-7.
- [CrossRef] [PubMed] [Google Scholar]
- Acral lentiginous melanoma, Indolent Subtype Diagnosed by en bloc excision: A case report. Ann Dermatol. 2017;29:327-30.
- [CrossRef] [PubMed] [Google Scholar]
- A clinicopathologic analysis of 177 acral melanomas in Koreans: Relevance of spreading pattern and physical stress. JAMA Dermatol. 2013;149:1281-8.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathological analysis of the progression pattern of subungual melanoma: Late tendency of dermal invasion in the nail matrix area. Mod Pathol. 2014;27:1461-7.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma staging: Evidence based changes in the American Joint Committee on Cancer eighth edition cancer staging manual In: CA Cancer J Clin. Vol 67. 2017. p. :472-92.
- [CrossRef] [PubMed] [Google Scholar]
- Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986 2005. Arch Dermatol. 2009;145:427-34.
- [CrossRef] [PubMed] [Google Scholar]
- Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135-47.
- [CrossRef] [PubMed] [Google Scholar]
- Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340-6.
- [CrossRef] [PubMed] [Google Scholar]
- Different patterns of cell proliferation and death and oncogene expression in cutaneous malignant melanoma. J Cutan Pathol. 1998;25:244-51.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of recurrence patterns in acral versus nonacral melanoma: Should histologic subtype influence treatment guidelines? J Natl Compr Canc Netw. 2014;12:1706-12.
- [CrossRef] [PubMed] [Google Scholar]
- Concomitant traumas influence prognosis in melanomas of the nail apparatus. Br J Dermatol. 2006;155:76-80.
- [CrossRef] [PubMed] [Google Scholar]
- Subungual melanoma. A review of 93 cases with identification of prognostic variables. Clin Orthop Relat Res. 2000;378:206-12.
- [CrossRef] [Google Scholar]
- Association between NRAS and BRAF mutational status and melanoma specific survival among patients with higher risk primary melanoma. JAMA Oncol. 2015;1:359-68.
- [CrossRef] [PubMed] [Google Scholar]
- Constitutive activation of the phosphatidyl inositol 3 kinase signalling pathway in acral lentiginous melanoma. Br J Dermatol. 2008;158:411-3.
- [CrossRef] [PubMed] [Google Scholar]
- Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006-14.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic, misdiagnosis, and survival differences between clinically amelanotic melanomas and pigmented melanomas. J Am Acad Dermatol. 2019;80:1292-8.
- [CrossRef] [PubMed] [Google Scholar]
- Subungual melanoma: Histological examination of 50 cases from early stage to bone invasion. J Dermatol. 2008;35:695-703.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin Exp Dermatol. 2004;29:600-4.
- [CrossRef] [PubMed] [Google Scholar]
- Melanoma: Differences between Asian and Caucasian patients. Ann Acad Med Singapore. 2012;41:17-20.
- [Google Scholar]
- Acral melanoma: A retrospective cohort from the Brazilian National Cancer Institute (INCA) Melanoma Res. 2018;28:458-64.
- [CrossRef] [PubMed] [Google Scholar]
- Acral lentiginous melanoma: Who benefits from sentinel lymph node biopsy? J Am Acad Dermatol. 2015;72:71-7.
- [CrossRef] [PubMed] [Google Scholar]






