Translate this page into:
The unprecedented epidemic-like scenario of dermatophytosis in India: II. Diagnostic methods and taxonomical aspects
Corresponding author: Dr. Resham Vasani, Bhojani Clinic, Earth Classic, Ground Floor, Babasaheb Ambedkar Road, Matunga, Mumbai, Maharashtra, India. dr.resham@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: II. Diagnostic methods and taxonomical aspects. Indian J Dermatol Venereol Leprol 2021;87:326-32.
Abstract
Trichophyton (T.) mentagrophytes now accounts for an overwhelming majority of clinical cases in India, a new “Indian genotype” (T. mentagrophytes ITS genotype VIII) having been isolated from skin samples obtained from cases across a wide geographical distribution in this country. The conventional diagnostic methods, like fungal culture, are, however, inadequate for diagnosing this agent. Thus, molecular methods of diagnosis are necessary for proper characterization of the causative agent. The shift in the predominant agent of dermatophytosis from T. rubrum to T. mentagrophytes, within a relatively short span of time, is without historic parallel. The apparent ease of transmission of a zoophilic fungus among human hosts can also be explained by means of mycological phenomena, like anthropization.
Keywords
Anthropization
phylogenetic tree
T. mentagrophytes ITS genotype VIII
Trichophyton mentagrophytes
Introduction
Chronic and recurrent dermatophytoses caused by Trichophyton (T.) mentagrophytes affects otherwise healthy people of all ages in India ranging from infants to the elderly. The clinical picture and distribution pattern of the tinea cruris, tinea corporis as well as tinea faciei is most often characteristic enough to diagnose clinically. However, detection of the mycological pathogen is essential, especially in view of the increasing terbinafine resistance of the dermatophytes involved. This includes conventional diagnostic tools like potassium hydroxide mount and fungal culture. Molecular techniques are also becoming increasingly important for confirmation of the species and genotype of the isolated dermatophyte in view of the various genotypes of the predominantly isolated T. mentagrophytes. ITS genotype VIII is most commonly isolated from Indian patients, with significant isolates showing mutations in the squalene epoxidase gene. It would be of great advantage to perform polymerase chain reaction (PCR) to detect dermatophyte DNA followed by sequencing in as many specialized centers as possible.
Diagnosis of Superficial Dermatophytosis
As mentioned earlier, the clinical features of tinea are manifold and confusion in clinical diagnosis does occur from time to time, especially in patients who have abused steroids containing fixed-dose combinations (FDCs). Indian dermatologists, both in private practice as well as a majority of academic departments, do not routinely perform potassium hydroxide (KOH) examination and order fungal culture even more rarely. The microscopic detection of fungal elements using conventional KOH preparations is easy to perform but has diminished sensitivity when compared to the much more sensitive fluorescent Calcofluor® or Blankophor® preparations.1 Both microscopic tests, however, share the disadvantage of lack of specificity in identifying fungal genus or species.
Clinical diagnosis has been the norm so far, and this is not surprising considering the number of patients, paucity of trained technical staff and infrastructure. It is only rather recently that dermatologists, more so in teaching departments, are keen to investigate, isolate and identify the etiological agent and know its antifungal susceptibility pattern for better management.2-4 The driving force for this change is the increasing number of chronic and relapsing or recurrent cases even after completion of adequate treatment and a recent upsurge in antifungal resistance.
Collection of quality samples is essential for efficient laboratory diagnosis of dermatophytosis. Certain parameters before sampling should be considered, such as removal of contaminant with 70% alcohol at the site of the lesion; and, collection of samples before starting antifungal treatment, or at least 15 days after stopping topical antifungal drugs or three months after stopping oral antifungals.5 These conditions are, however, not practical and possible in most instances in India. Skin sample should be collected from the edge of the lesion and hair samples (hair roots) are plucked from the basal area.5
Conventional Methods
Direct microscopic examination is a simple screening technique to allow the clinician to start antifungal therapy for dermatophytosis. Direct microscopic identification requires a clearing agent (10 or 20% KOH with or without dimethyl sulfoxide/ Amann’s chloral lactophenol, / sodium dodecyl sulfate) to digest keratin from the sample.5 Culture is important to know the etiological agent responsible for dermatophytosis. T. mentagrophytes is increasingly being isolated from chronic recalcitrant dermatophytosis in India.2,4 Scrapings from centrifugal lesions of the free skin should be cultured on common mycological agar media such as Sabouraud´s dextrose agar (SDA), and additionally on cycloheximide (Actidion®) containing SDA which suppresses growth of other saprophytes (molds) and yeasts which are found as commensals on the skin. Alternatively, Dermasel® agar (Oxoid), which also contains cycloheximide and chloramphenicol, might be used for primary cultivation. Fungal cultures should be incubated at 28°C for four weeks. The cultures are to be inspected visually for fungal growth twice weekly. Initial conventional identification of the dermatophytes is based on macroscopic features (colony growth in culture) and their micromorphology using Lactophenol Cotton Blue preparation.1
Cultural features of Trichophyton mentagrophytes
One should understand the limitation of the culture-based methods in the present scenario. The culture positivity rate even in smear-positive specimens (resembling dermatophytes) is not more than 65–75%.6 Flat, white, fast-growing and expanding colonies with hyphae bundles in the periphery and slight yellowish-to-beige pigmentation in the center are typical for T. mentagrophytes of the Indian genotype [Figures 1a and b]. The reverse side of the colonies shows yellowish-to-beige and brown pigmentation [Figures 1c and d]. It is nearly impossible to distinguish the different genotypes of the T. mentagrophytes/T. interdigitale complex based on morphology of the fungal culture. In contrast, T. mentagrophytes of the genotype VII (from Thailand) exhibits typical brown-red stained reverse sides of colonies in primary culture,7 which is markedly different from the Indian genotype of T. mentagrophytes. Other T. mentagrophytes genotypes, however, do not form such clear features. T. interdigitale (generally considered as anthropophilic form of T. mentagrophytes) and T. mentagrophytes (generally considered as zoophilic form of T. mentagrophytes) cannot be differentiated on the basis of cultural characteristics and biochemical reactions.8 In all this confusion it is observed that T. mentagrophytes grows much quicker than T. rubrum.1
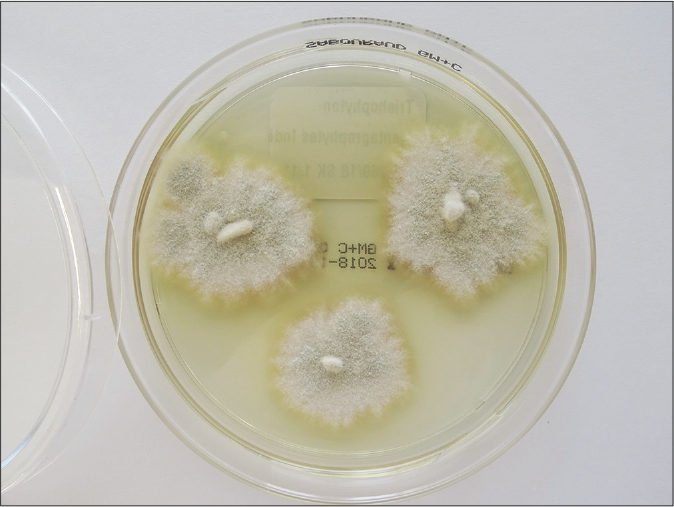
- Flat, white, slightly yellowish, fast growing and expanding granular colonies with hyphae bundles in the periphery and with slight yellowish-to-beige pigmentation in the centre are typical for Trichophyton mentagrophytes of the Indian genotype VIII. One-week old colonies on Sabouraud’s dextrose agar
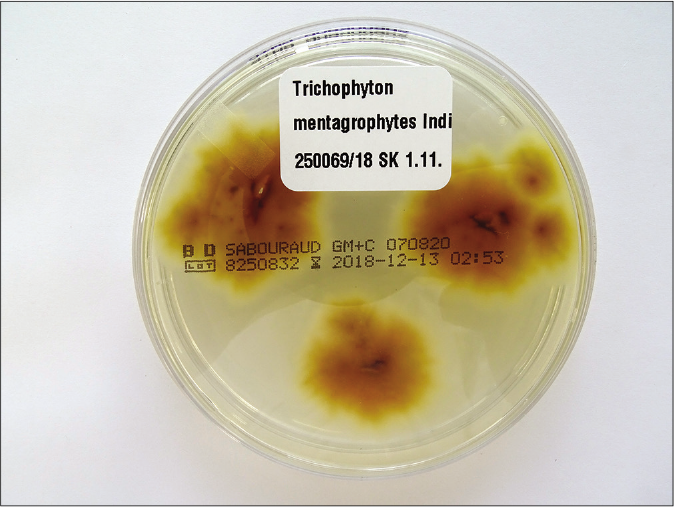
- Brown-yellowish pigmented reverse side of fungal colonies of Trichophyton mentagrophytes from Figure 1a
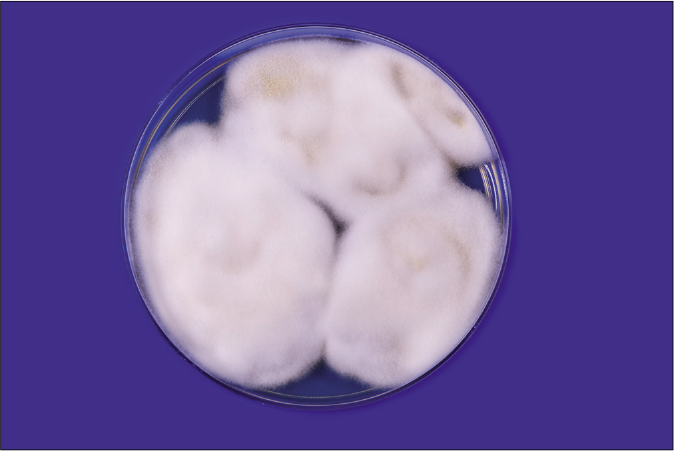
- White and slightly yellowish fluffy fast growing colonies of Trichophyton mentagrophytes VIII India
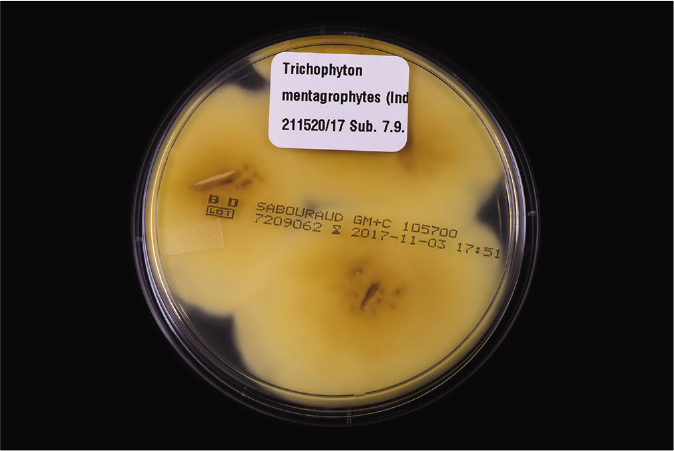
- Yellow to bright brown pigmentation of the reverse side of colonies from fungal culture of Figure 1c
Microarchitecture of Trichophyton mentagrophytes complex
Microscopic Lactophenol Cotton Blue preparations performed from cultural material reveal both small and big round microconidia together with chlamydospores of different sizes and cigar-shaped macroconidia. Spiral hyphae are formed in older cultures. Unfortunately, these microscopic features are not species-specific and one can find them in all other T. mentagrophytes genotypes including T. interdigitale.
Nonmycological tests for dermatophytoses
Diagnosis of dermatophytosis is based on conventional and molecular mycological methods. Non-mycological investigations like dermoscopy in case of tinea capitis, and Wood’s lamp examination in case of suspected Microsporum sp., and T. schoenleinii infections, are available, and can be used.9 However, the use of Wood’s lamp examination in the current scenario is irrelevant.
A few reports of dermoscopic findings of dermatophytosis are available and may be read by those interested. But, until now, dermoscopy has been found useful in rapid diagnosis of tinea capitis. It was shown that dermoscopy and calcium fluorescent microscopy are highly efficient and precise diagnostic tools in tinea capitis.10 Comma and corkscrew hairs are the two most specific dermoscopic patterns of tinea capitis.11 As far as the evidence goes, however, dermatoscopy is as irrelevant in diagnosing tinea corporis and tinea cruris, given its nonspecificity, as Wood’s lamp. Recently, dermoscopy was used for tinea incognita resulting from corticosteroid abuse.12 Morse-code hairs, deformable and translucent, comma and cork screw hairs, and perifollicular scaling were significant findings13 in this Indian and Iranian study. The problem remains, however, the impossible species identification which is necessary in chronic recalcitrant dermatophytosis. Similar results were shown based in a first pilot study of dermoscopy in 100 Indian patients with tinea corporis, tinea cruris, and tinea capitis.14 But, dermoscopic findings were not correlated to fungal culture in this study.
Molecular Methods
Molecular techniques are being increasingly employed in the clinical microbiological laboratories for identification and direct detection of organisms in clinical specimens because of high sensitivity and specificity and small turnaround time. The nucleic acid-based method depends on the detection of genotypic characteristics in pathogenic organisms. These are more specific than those based on phenotypic characters because genotypic characteristics are less affected by external environment.15 Rapid diagnosis is required to achieve successful treatment for dermatophytosis. Many dermatophytes are non-sporulating in culture media and are difficult to identify by the conventional method, and identification by molecular technique is very useful in such cases.1
Isolation of DNA from the clinical samples and culture growth is performed by using phenol- chloroform method or commercially available DNA extraction kits. Polymerase chain reaction (PCR) and modified PCR are widely used tools for detection of molecular characteristics of an organism. Identification of an organism is achieved by using a variety of primers such as single species-specific primer, pandermatophyte primer or pan-fungal primer which target the internal transcribed region (ITS), 28S rDNA region, and gene encoding topoisomerase II, chitin synthase I, beta-tubulin, calmodulin, translation elongation factor etc.16,17
MALDI TOF (matrix-assisted laser desorption/ionization time of flight) mass spectroscopy may be used as a culture confirmation method.18 The specificity, of this spectroscopic method, however, is not high enough to enable differentiation between different genotypes within the species T. mentagrophytes. It was shown using MALDI-TOF mass spectrometry that phylogroups of T. mentagrophytes were often cross-identified to one another or to T. interdigitale.19
Taxonomy of Dermatophytes
For confirmation and definitive identification of the dermatophyte species and distinct ITS genotype, sequencing of the ribosomal DNA (rDNA), mainly targeting ITS 1, 5.8S, and ITS 2 regions, and the translation elongation factor (TEF)-1α gene, should be performed for all strains suspected as the Indian genotype of T. mentagrophytes.20 First, DNA from fungal cultures has to be extracted.20 This is followed by PCR amplification of a ~ 900 bp DNA fragment using universal primers that bind to flanking pan-fungal sequence regions. The following primers are used for sequencing of the ITS region of the rDNA: V9G 5’-TTACGTCCCTGCCCTTTGTA-3’ and LSU266 5’-GCATTCCCAAACAACTCGACTC-3’. The length of the analyzed region in the TEF-1α gene varies from 709 to 769 nucleotides among the various dermatophyte species. Primers EF1a-F 5’CACATTAACTTGGTCGTTATCG 3’ and EF1a-R 5’CATCCTTGGAGATACCAGC3’ are used for sequencing TEF-1α gene.21
Phylogenetic tree of Trichophyton mentagrophytes genotype VIII and other genotypes of the species
The phylogenetic analysis of the dermatophytes – the dendrogram of fungal strains – based on ITS region of the rDNA and of the TEF 1-α-gene allows genetic differentiation between T. mentagrophytes and the closely related species T. interdigitale, and additionally the distinction of all newly described genotypes within the species T. mentagrophytes [Figure 2].22,23 The species and genotypes can be clearly distinguished from one another by this analysis. Sequencing of the TEF-1α gene, however, does not allow better differentiation of T. mentagrophytes and T. interdigitale genotypes when compared to sequencing of the ITS-2-region.
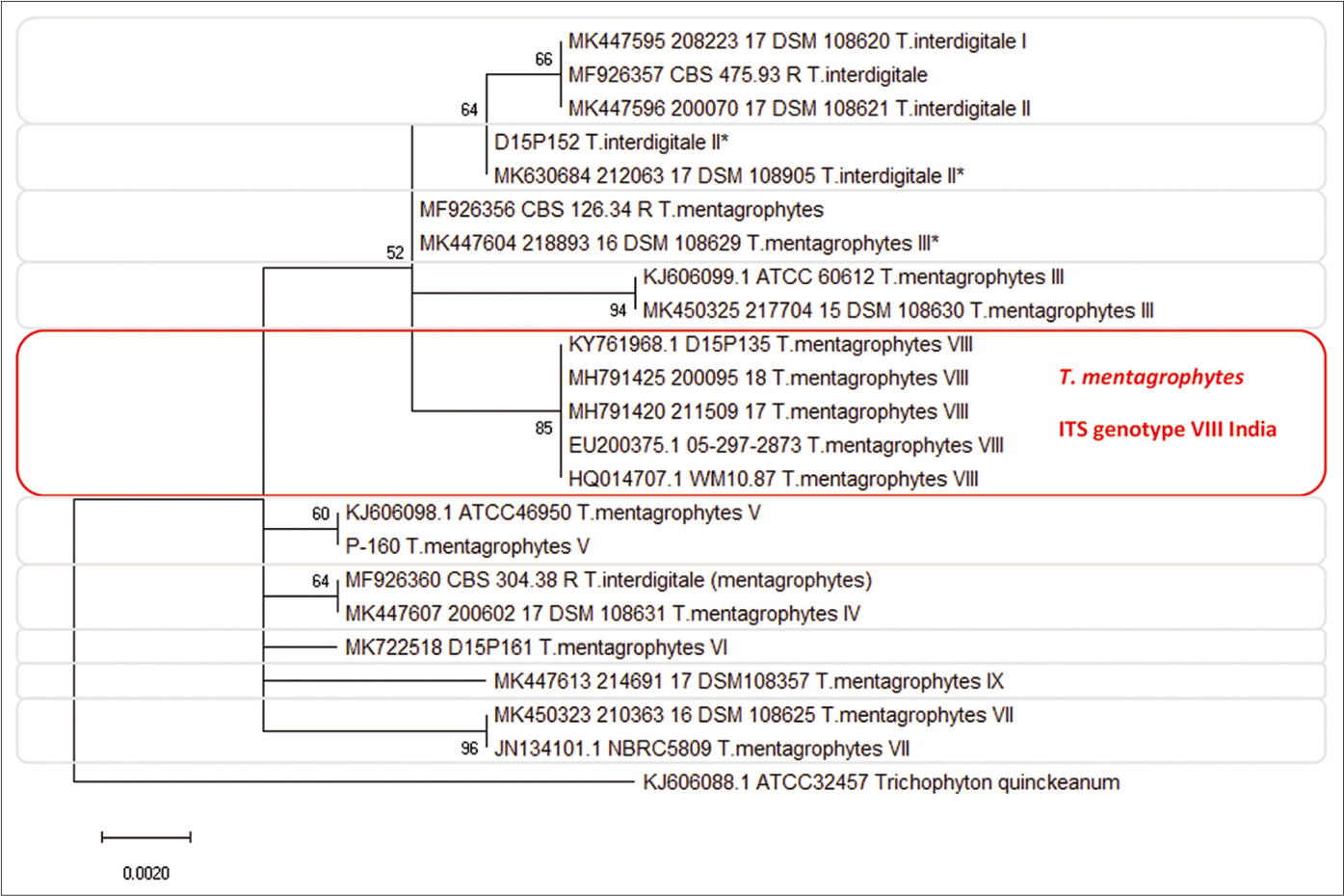
- Phylogenetic tree of Trichophyton mentagrophytes based on sequencing of the ITS rDNA region of ribosomal DNA. The new ITS genotype VIII (Indian variant) of Trichophyton mentagrophytes is forming an own cluster which can clear be distinguished from all other Trichophyton mentagrophytes genotypes, e.g. genotype II, III*, V, and VII (Thai variant). Rooted with Trichophyton quinckeanum. Statistical Method: Evolutionary analysis by maximum likelihood method. The evolutionary history was inferred by using the maximum likelihood method and Tamura-Nei model,22 and evolutionary analyses were conducted in MEGA X23
Genotyping of Trichophyton mentagrophytes
Based on ITS sequence, currently at least 10 genotypes of T. mentagrophytes (TM) and T. interdigitale (TI) are described by performing sequencing of the ITS region of the rDNA. These genotypes are mostly associated to the geographic origin or to the source of infection. Currently, two genotypes of TI (ITS Type I [Europe] and ITS Type II [Cosmopolite]), were described. TM, however, comprises the common zoophilic “German” ITS Type III (Europe) and III* (Cosmopolite), genotype IV (UK, USA, South Africa and France), V (Asia, Egypt, Iran, Iraq and Japan), VI (Europe, Russia and Finland), VII (from Thailand originating isolates in Switzerland and Germany, USA, Australia, Georgia, Russia, and Vietnam), VIII (Asia, India, Iran, Oman, and Australia), and last but not least, the Australian genotype (ITS Type IX*).
Trichophyton mentagrophytes – Principal causative agent of superficial dermatophytosis in India
Recently, mycological and molecular diagnostics of the causative agent of the epidemic of chronic and recurrent dermatophytosis in India have shown a marked change. The predominant species has changed from T. rubrum to T. mentagrophytes as is mentioned in the epidemiology section. A novel multicentric effort was able to demonstrate that 93.21 % of the dermatophytes isolated from patients with recurrent tinea cruris and tinea corporis in India were T. mentagrophytes24 and only 6.79 % were T. rubrum. It is noteworthy that according to the latest suggested classification and new taxonomy of dermatophytes, the former ‘T. mentagrophytes complex’ (TMC) is now subdivided into T. mentagrophytes (zoophilic strains) and T. interdigitale (anthropophilic strains). According to this currently accepted consensus classification, the species identified as T. interdigitale in some studies on the chronic, relapsing dermatophytoses in India is factually considered to be T. mentagrophytes and not T. interdigitale.4,25 Ironically the taxonomy is rather unclear due to genotypic and phenotypic similarities. Two Indian studies have reported T. interdigitale as a major cause of the ongoing epidemic in India, based on the ITS sequences and blast results from an online database (NCBI, CBS database).2,4,26 However, Nenoff et al. have analyzed the T. interdigitale sequences mentioned above and have identified them as a zoophilic species of T. mentagrophytes (Type VIII)27 and not T. interdigitale. Recently, analysis of multigene sequences like ITS, beta-tubulin, translation elongation factor and calmodulin gene revealed that all the T. mentagrophytes strains of India were distinct from other clades. Whole-genome sequencing of 20 Indian isolates showed that they share similarities with the ancestor T. mentagrophytes/interdigitale complex, but could not conclusively differentiate between these two species.27,28
As of now, more evidence is required to conclude that these Indian strains are a new species. The identification of a distinct genotype VIII of T. mentagrophytes in 2018 which finds a mention in respectable publications following the first description, is as per the current classification last updated in 2017 and would remain so until a new classification becomes available.16,29,30 Recently it was also found to have unique Topoisomerase 2 (TOP2) sequence type, underlining the fact that this distinct Indian ITS and TOP2 genotype are part of the T. mentagrophytes clade and not a ‘cryptic’ new species.31 A striking resemblance to this genotype has also been found in those demonstrated in Iran and Oman.32 This particular study performed in 2016 also referred to the strains as T. interdigitale, because it followed the former taxonomy. However, the strains are now identified as T. mentagrophytes Type VIII as per the revised taxonomy.
Trichophyton mentagrophytes Genotype VIII, India or Trichophyton indotineae?
Recently, two strains of Trichophyton mentagrophytes Genotype VIII India, originating from India and Nepal, have been isolated in Japan. Kano et al33. have described these two strains as the new dermatophyte species Trichophyton indotineae. From our point of view and understanding of current established dermatophyte taxonomy, the T. mentagrophytes Type VIII is only one variety within the cluster of a large number of genotypes of the T. mentagrophytes / T. interdigitale complex. Therefore, it does not appear justifiable to attempt assigning this single genotype VIII of T. mentagrophytes to a brand new species.
Probable reason for epidemiological shift of etiological agent
A major epidemiological shift in etiological agents, similar to what is seen in India today, was reported in the Western world in 1954. Increase in incidence of T. rubrum was observed from 1.8% in 1935 to 10% in 1954, whereas, the incidence of T. mentagrophytes reduced from 80% 1935 to 20%.34 Many hypotheses were proposed to explain this epidemiological shift which included environmental factors (humidity, temperature shift, trauma) and many internal factors like a host-parasite relationship, host susceptibility, and immunological factors.34 Recently, Nenoff et al. suggested that the rather abrupt shift in epidemiology in India could well be due to the rampant and irrational use of topical corticosteroids (clobestasol propionate, betamethasone dipropionate, beclomethasone dipropionate, and others, mixed with antibiotics and antifungals), easily available over the counter in India. It is presumed that the topical steroid, topical antifungal and antibacterial agents may alter the microbiological milieu of the skin and its microbiome, thus providing a favorable condition for the survival of T. mentagrophytes. However, further studies are needed to substantiate this hypothesis.24
Several factors including host immune system, environmental factors, as well as topical corticosteroid abuse, are suggested to be responsible for the increase in the incidence and prevalence of chronic recalcitrant tinea in India involving the shift in the species. While nothing dramatic seems to have changed in the host immune system or environmental factors, the association of ‘widespread topical steroid abuse’ with the epidemic like scenario involving shift in species is an observation that cannot be wished away and warrants documentation. Its temporal association with the burgeoning FDC cream market, especially products containing clobetasol propionate, makes the proposed connection a compelling one in the opinion of the authors.35-39 The significant increase in the incidence and prevalence of dermatophytosis, especially the extensive tinea cruris, tinea corporis and tinea faciei, are associated with the emergence of a hitherto undescribed genotype of T. mentagrophytes which exhibits a high level of adaptation to a novel skin climate against the backdrop of local immune suppression and very likely an altered cutaneous microbiome. Needless to say, that this speculation advanced by authors needs further studies, awaits further evidence.
Mode of transmission
Interestingly, the transmission in India occurs almost exclusively among human beings. Evidence is lacking till date for transmission of this particular genotype of T. mentagrophytes to occur from animals like cats, dogs, other pets, rodents or cattle, to humans as would be expected. Household pets are relatively uncommon in India, unlike in the Western world. A combination of cultural attitudes, inadequate space, and resources may also be responsible for this. A phenomenon that merits mention in the current context is of “anthropization”. This refers to the mechanism wherein certain dermatophytes adapt to the surface of human skin. The phenomenon forms the core of evolution of hitherto geophilic or zoophilic fungi into anthropophilic ones. The phenomenon has been described in the case of previously geophilic T. rubrum in becoming anthropophilic.24 Anthropization can therefore explain the previously zoophilic T. mentagrophytes to have become anthropophilic, resulting in the unusual behavior of human-to-human transmission.24
It is emphasized that the identification of T. mentagrophytes (type VIII, India), is not based on its biological behavior of its transmissibility from animals to human beings. Rather, it is the DNA-sequencing of the fungus that forms the basis for its identification as part of the T. mentagrophytes species clade.40
T. mentagrophytes, an innately zoophilic fungus is frequently isolated from animals, in particular rodents, but also from cats and dogs.41 However, interestingly two specific ITS genotypes of T. mentagrophytes, i.e. Type VII (Thai variant) and VIII (India) exhibit anthropophilic behavior of human to human transmission.31 T. mentagrophytes Type VII (Thai variant) is genetically within the clade of T. mentagrophytes, and bears a morphological similarity too. However its transmission in abscessing tinea pubogenitalis does not occur from animals, The fungus is transmitted, without exception, from human to human as an agent of what is now considered a sexual transmitted infection.7,42-44
Conclusion
Diagnosis of chronic recalcitrant dermatophytosis, e.g. tinea cruris, tinea corporis, and tinea faciei, especially the chronic widespread variants, should be confirmed using conventional mycological diagnostics. Microscopic preparation, but more importantly, cultural detection of the causative dermatophyte species – T. mentagrophytes, and in some cases, T. rubrum and others should be performed according to the recommendations of national and international guidelines. For epidemiological purposes, molecular methods: PCR and DNA sequencing for identification of the exact genotype within the species T. mentagrophytes are necessary. Preliminary results show a marked change among the causative agents of dermatophytoses in India. The predominant species has changed from T. rubrum to T. mentagrophytes. PCR followed by DNA sequencing identifies it as T. mentagrophytes ITS genotype VIII. Even though T. mentagrophytes is a zoophilic dermatophyte, this particular Indian genotype is being transmitted from human being to human-like an anthropophilic dermatophyte.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mycology-an update part 2: Dermatomycoses: Clinical picture and diagnostics. J Dtsch Dermatol Ges. 2014;12:749-77.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother. 2018;62:e01038-18.
- [CrossRef] [PubMed] [Google Scholar]
- Mutation in the squalene epoxidase gene of Trichophyton interdigitale and trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522-17.
- [CrossRef] [PubMed] [Google Scholar]
- High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477-84.
- [CrossRef] [PubMed] [Google Scholar]
- Overview and update on the laboratory diagnosis of dermatophytosis. Clin Dermatol Rev. 2017;1:3.
- [CrossRef] [Google Scholar]
- Development and evaluation of a pandermatophyte polymerase chain reaction with species-level identification using sloppy molecular beacon probes. Br J Dermatol. 2019;180:1489-97.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea barbae profunda due to Trichophyton mentagrophytes after journey to Thailand: Case report and review. Hautarzt. 2017;68:639-48.
- [CrossRef] [PubMed] [Google Scholar]
- Trichophyton mentagrophytes sive interdigitale? A dermatophyte in the course of time. J Dtsch Dermatol Ges. 2007;5:198-202.
- [CrossRef] [PubMed] [Google Scholar]
- 7-year-old male with suppurative, abscess-forming, pressure painful lesions of the scalp: Preparation for the medical specialist examination: Part 14. Hautarzt. 2018;69:136-44.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea capitis: Dermoscopy and calcium fluorescent microscopy as highly efficient and precise diagnostic tools. An Bras Dermatol. 2020;95:332-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy-A simple and rapid in vivo diagnostic technique for tinea incognito. An Bras Dermatol. 2019;94:612-4.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of terbinafine 500 mg once daily in patients with dermatophytosis. Indian J Dermatol. 2017;62:395-9.
- [CrossRef] [PubMed] [Google Scholar]
- Can dermoscopy serve as a diagnostic tool in dermatophytosis? A pilot study. Indian Dermatol Online J. 2019;10:530-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of the DermaGenius® Nail real-time PCR assay for the detection of dermatophytes and Candida albicans in nails. Med Mycol. 2019;57:277-83.
- [CrossRef] [PubMed] [Google Scholar]
- Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182:5-31.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular taxonomy of dermatophytes and related fungi by chitin synthase 1 (CHS1) gene sequences. Antonie Van Leeuwenhoek. 2003;83:11-20.
- [CrossRef] [PubMed] [Google Scholar]
- MALDI-TOF mass spectrometry-a rapid method for the identification of dermatophyte species. Med Mycol. 2013;51:17-24.
- [CrossRef] [PubMed] [Google Scholar]
- Multilocus phylogeny of the Trichophyton mentagrophytes species complex and the application of matrix-assisted laser desorption/ionization-time-of-flight (MALDITOF) mass spectrometry for the rapid identification of dermatophytes. Mycologia. 2018;110:118-30.
- [CrossRef] [PubMed] [Google Scholar]
- Do the new molecular assays-microarray and realtime polymerase chain reaction-for dermatophyte detection keep what they promise? Hautarzt. 2019;70:618-26.
- [CrossRef] [PubMed] [Google Scholar]
- Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med Mycol. 2015;53:215-24.
- [CrossRef] [PubMed] [Google Scholar]
- Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512-26.
- [Google Scholar]
- MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547-9.
- [CrossRef] [PubMed] [Google Scholar]
- The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes-A molecular study. Mycoses. 2019;62:336-56.
- [CrossRef] [PubMed] [Google Scholar]
- Perspectives on misidentification of Trichophyton interdigitale/Trichophyton mentagrophytes using internal transcribed spacer region sequencing: Urgent need to update the sequence database. Mycoses. 2019;62:11-5.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular strain typing of Trichophyton mentagrophytes (T. mentagrophytes var. interdigitale) using non-transcribed spacer region as a molecular marker. Indian J Med Res. 2017;146:636-41.
- [Google Scholar]
- A clarion call for preventing taxonomical errors of dermatophytes using the example of the novel Trichophyton mentagrophytes genotype VIII uniformly isolated in the Indian epidemic of superficial dermatophytosis. Mycoses. 2019;62:6-10. Comment on: “High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene.” by Ashutosh Singh et al., published in Mycoses 2018;61:477-84
- [CrossRef] [PubMed] [Google Scholar]
- A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: Genomic insights and resistance profile. Fungal Genet Biol. 2019;133:103266.
- [CrossRef] [PubMed] [Google Scholar]
- A hundred years of diagnosing superficial fungal infections: Where do we come from, where are we now and where would we like to go? Acta Derm Venereol. 2020;100:adv00111.
- [CrossRef] [PubMed] [Google Scholar]
- Species boundaries in the Trichophyton mentagrophytes/T. interdigitale species complex. Med Mycol. 2019;57:781-9.
- [CrossRef] [PubMed] [Google Scholar]
- DNA topoisomerase 2 gene polymorphism in dermatophytes. Mycoses. 2020;63:694-703.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological aspects of dermatophytosis in Khuzestan, Southwestern Iran, an update. Mycopathologia. 2016;181:547-53.
- [CrossRef] [PubMed] [Google Scholar]
- Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185:947-58.
- [Google Scholar]
- The etiology of dermatophytosis; shift from Trichophyton mentagrophytes to Trichophyton rubrum, 1935-1954. AMA Arch Derm. 1957;75:66-9.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:250-1.
- [CrossRef] [Google Scholar]
- Topical corticosteroids abuse: A clinical study of cutaneous adverse effects. Indian J Dermatol. 2017;62:675.
- [CrossRef] [PubMed] [Google Scholar]
- Surrogate advertisement of a super-potent corticosteroid-containing cream: An alarming development and a cautionary tale of its consequences. Indian J Dermatol Venereol Leprol. 2019;85:104-6.
- [CrossRef] [PubMed] [Google Scholar]
- Emergence of recalcitrant dermatophytosis in India. Lancet Infect Dis. 2018;18:718-9.
- [CrossRef] [Google Scholar]
- Trichophyton mentagrophytes and T. interdigitale genotypes are associated with particular geographic areas and clinical manifestations. Mycoses. 2019;69:1084-91.
- [CrossRef] [PubMed] [Google Scholar]
- Mycology-an update. Part 1: Dermatomycoses: Causative agents, epidemiology and pathogenesis. J Dtsch Dermatol Ges. 2014;12:188-209.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea genitalis: A new entity of sexually transmitted infection? Case series and review of the literature. Sex Transm Infect. 2015;91:493-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tinea genitalis profunda due to Trichophyton mentagrophytes after a Journey to Egypt. Akt Dermatol. 2017;43:146-53.
- [CrossRef] [Google Scholar]
- Trichophyton mentagrophytes ITS genotype VII from Thailand as causative pathogen of abscessing dermatophytoses in Germany In: Dermatophytes and Dermatophytoses. Mycopathologia. e-book, Springer Edition, Edited by Jean-Philippe Bouchara, Pietro Nenoff, Aditya Gupta and Vishnu Chaturvedi 2021. In press
- [Google Scholar]






