Translate this page into:
Artificial intelligence in dermatology and healthcare: An overview
Corresponding author: Dr. Varadraj Vasant Pai, Department of Dermatology, Venereology and Leprosy, Goa Medical College, Bambolim, Goa, India. docpai@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Pai VV, Pai RB. Artificial intelligence in dermatology and healthcare: An overview. Indian J Dermatol Venereol Leprol 2021;87:457-67.
Abstract
Many aspects of our life are affected by technology. One of the most discussed advancements of modern technologies is artificial intelligence. It involves computational methods which in some way mimic the human thought process. Just like other branches, the medical field also has come under the ambit of artificial intelligence. Almost every field in medicine has been touched by its effect in one way or the other. Prominent among them are medical diagnosis, medical statistics, robotics, and human biology. Medical imaging is one of the foremost specialties with artificial intelligence applications, wherein deep learning methods like artificial neural networks are commonly used. artificial intelligence application in dermatology was initially restricted to the analysis of melanoma and pigmentary skin lesions, has now expanded and covers many dermatoses. Though the applications of artificial intelligence are ever increasing, large data requirements, interpretation of data and ethical concerns are some of its limitations in the present day.
Keywords
Artificial intelligence
deep learning
dermatology
machine learning
medical imaging
Introduction
Machines have been used by man from time immemorial in his pursuit to survive and make life easier. The evolution of machines runs parallel to human spirit and enterprise, and has evolved from simple tools to modern-day computers. Dependence on machines has penetrated almost every aspect of human life including medicine. While the advent of modern medicine noted a reliance on subjective human skill, it has been gradually progressing towards a more objective approach with newer technological innovations.1 One of the most discussed advancements of modern technologies in recent times is artificial intelligence and the implication of its application in various domains.2 Artificial intelligence experts in medicine consider artificial intelligence to be the stethoscope of the 21st century.1
John McCarthy coined the term artificial intelligence in 1956 and defined it as “the science and engineering of making intelligent machines, especially intelligent computer programs.”3,4 The term applies to a broad range of items in medicine such as robotics, medical diagnosis, medical statistics, and human biology.5
History of Artificial Intelligence
The history of artificial intelligence can be believed to have begun in antiquity, with myths and stories of artificial beings designed by master craftsmen endowed with intelligence or consciousness.6 In the first millennium Before the common era, Indian, Chinese and Greek philosophers developed structured methods of formal deduction like syllogisms (three-part deductive reasoning), described in the ‘Nyaya school of thought’ and Aristotle’s work, which later moved towards Ramon Llull’s theory of a reasoning machine in 1300 Common era.2,7
In 1950, Alan Turing, one of the founders of modern computer science, propounded the idea of artificial intelligence and devised the Turing test, which is a machine’s ability to exhibit intelligent behaviour equivalent to, or indistinguishable from that of a human.8 Over the next few decades, algorithms were generated for mathematical problems and geometrical equations. With exponential gains in computer processing power and storage ability, software giants used artificial intelligence algorithms to understand consumer behaviour, to develop computer vision, natural language processing and robotics.6 Recently Google developed artificial intelligence programs namely Alpha Go and Alpha Zero, which were able to master the rules of an ancient Chinese game Go and chess by only playing against itself and then were able to defeat the best players in that sport.9,10 Artificial intelligence ended the human domination over scientific discoveries when a robot called Adam in 2007 was used to identify the function of a yeast gene.11
History of the Use of Artificial Intelligence in Healthcare
Artificial intelligence in medicine coincides with the advent of artificial intelligence in the modern era.2 Artificial intelligence has been applied in the field of medicine since as early as the 1950s when physicians made the first attempts to improve their diagnosis using computer-aided programs.11,12 The application of artificial intelligence technology in the field of surgery was first successfully investigated by Gunn in 1976 when he explored the possibility of diagnosing acute abdominal pain with computer analysis.7
The last few decades have seen a surge in the interest in both the virtual and physical branches of artificial intelligence in medicine. The virtual branch includes electronic health records, medical imaging and active guidance of physicians in their treatment decisions. The physical branch is best represented by robots used to assist the patient or the doctor.5,7 The market value of artificial intelligence in medicine is projected to reach $ 6.6 billion by 2021.13
The predominant fields of artificial intelligence with applications in medicine include: a) Machine learning including deep learning, b) natural language processing which includes content extraction, machine translation, question answering and text generation, c) visual applications which includes image recognition and machine vision, d) speech, and e) robotics.3,14,15
Artificial Intelligence, Machine Learning and Deep Learning
Arthur Samuel coined the term ‘machine learning’ defining it as, “the ability to learn without being explicitly programmed.” [Figure 1] In machine learning, instead of coding software with specific instructions to accomplish a particular task, the algorithm self-trains so that it can learn patterns by studying data directly.16,17 Machine learning technology powers many aspects of modern society: from email spam filters, search suggestions, online shopping suggestions, and speech recognition in smartphones, etc.18 Its ability to perform comprehensive analysis even with massive amounts of nonlinear data makes it favourable in medical decision-making.17,19
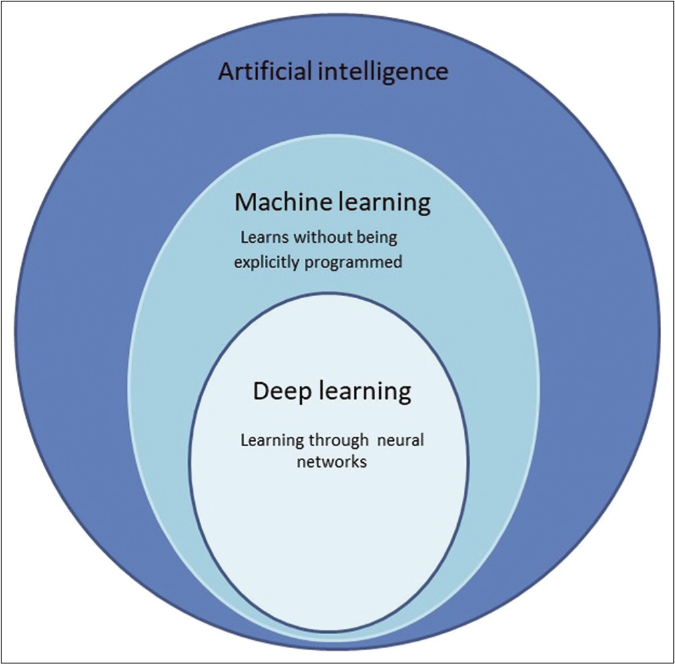
- Artificial intelligence is an all-encompassing term which includes machine learning and deep learning as one of it types and subtypes respectively
Machine learning tasks are classified into two broad categories, depending on the type of task: supervised and unsupervised. Supervised learning involves an algorithm working with labelled training data. It involves the categorisation of data and programming of the relationship between input and output data. In unsupervised learning, the algorithm identifies hidden patterns in a stack of data and the various outcomes.2,20,21 The difference between them is given in Table 1.
| Supervised learning | Unsupervised learning |
|---|---|
| Input data is labelled | Input data is unlabelled |
| Uses training dataset | Uses only input dataset |
| Learns known pattern | Learns unknown pattern |
| Used for prediction | Used for analysis |
| Eg., Algorithm for classification include decision trees network, support vector machines, bayesian network Algorithm for regression include logistic regression, neural network |
Eg., Cluster analysis, dimension reduction, association |
| Input » training » output | Input » output |
In medicine, supervised learning is often performed in medical imaging and when observations have labels and these observations are paired to associated features such as age, sex, or clinical variables.22,
Algorithms
In image analysis through machine learning, several types of classification methods or algorithms are mentioned in literature. Commonly used among these methods are the artificial neural network, support vector machine, decision trees, k-nearest neighbour, regression analysis classifiers, Bayesian network, random forest, discriminant analysis, etc.23 The neural network and support vector machines are the most common machine learning algorithms used in medical literature for image analysis. In data analysis of electronic health records, natural language processing is used.24
Neural networks
Artificial neural networks are a subset of machine learning, based on algorithms that are designed to recognize patterns that are inspired by the human neural network [Figure 2].25 An artificial neural network is structured as one input layer of neurons, one or more “hidden layers” and one output layer. The input data is processed through a large number of highly interconnected elements, which are called neurons or nodes.20 Deep learning is a subset of artificial neural network with stacked neural networks composed of one input and one output layer, and more than one hidden layer [Figure 3]. Deep learning algorithms require advanced computation and very large data.25,26
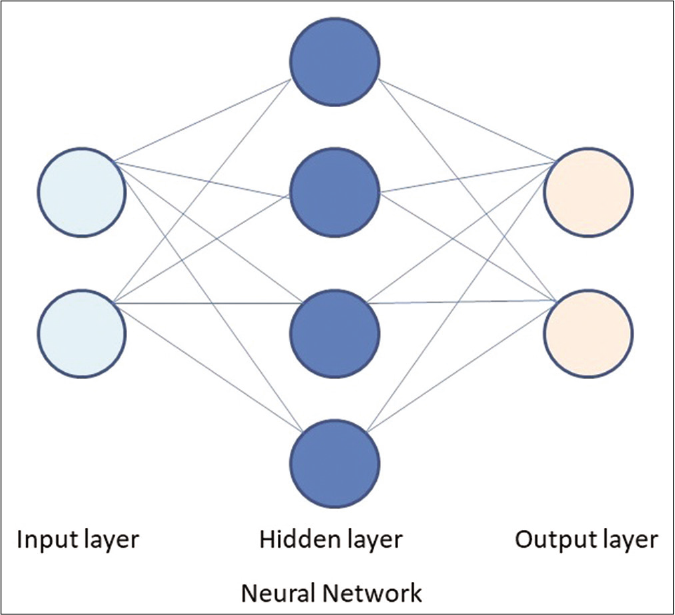
- Basic structure of a neural network with a input layer, hidden layer and output layer
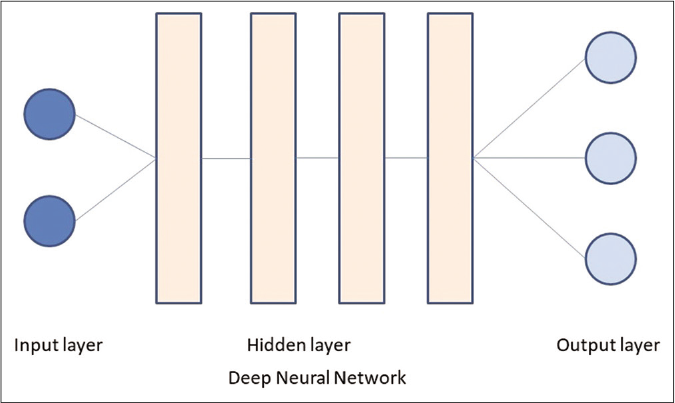
- The deep neural network differs from a basic neural network in having more than one hidden layers
Deep neural networks can be divided into normal (one dimensional) or convoluted (two or three dimensions). Convolutional neural network has gained importance in medical image analysis for extracting patterns from images.20 Convolutions are mathematic operations that are applied to pixel data in finding or filtering patterns.20,21 Convolutional neural network is typically composed of three types of layers (or building blocks): convolution, pooling, and fully connected layers. The convolution and pooling layers perform feature extraction, whereas the fully connected layer maps the extracted features into final output, such as classification [Figure 4].27
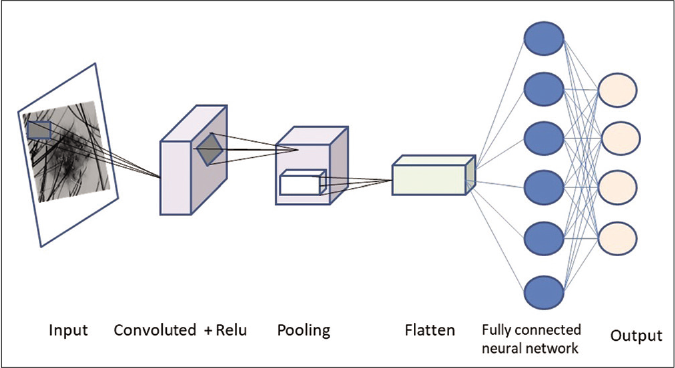
- Processing of image through a convoluted neural network
Each input image will pass it through a series of convolution layers with filters.,- Each layer learns to recognize a specific feature. For example: Once the first layer has successfully recognized a feature like an edge, it is fed to the next layer which trains itself to recognize more complex patterns like a corner in an image.The pooling (or down sampling) layer is used to reduce the spatial dimensions to gain computational performance As the model is repeatedly trained, individual convolutions begin to identify a specific portion of the image. Hundreds of these classifiers can be linked together to identify more complex structures within each image.20,21,27
Support vector machine
Support vector machine is a classification and regression algorithm for data classification in two classes that uses machine learning to maximize predictive accuracy while avoiding overfitting of data. It gives accuracy comparable to sophisticated neural networks when it is used in image analysis.28,29 The support vector machine classifier is constructed by projecting training data into a higher dimensional space known as a hyperplane, which maximizes the separation between the classes [Figure 5].30
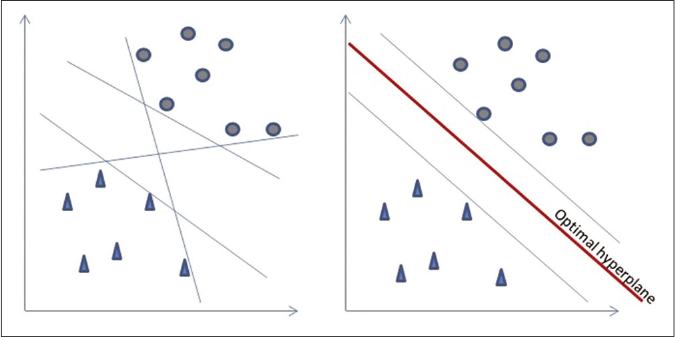
- Support vector machine algorithm
Natural language processing
Natural language processing is a machine learning program concerned with the use of software programming to understand and manipulate natural language text or speech for practical purposes.2 It is used to extract information from unstructured data such as electronic health records electronic health records, which includes physical examination, clinical laboratory reports, operative notes and discharge summaries or medical journals. Data entry tasks of electronic health records takes time away from patient care and is contributing largely to physician burnout. The natural language processing procedures target at turning texts to machine-readable structured data, which can then be analysed by other machine learning techniques.26,31,32
Computer vision
Computer vision is a field in imaging which aims at designing systems mimicking the human sense of sight. This field involves areas such as artificial intelligence, digital image processing, machine learning, deep learning, pattern recognition, and scientific computing. The applications of computer vision include medical uses like lesion or cells classification and tumor detection, 2D/3D segmentation, 3D human organ reconstruction (MRI or ultrasound), vision-guided robotics surgery or robotics: for localisation, navigation, manipulation and human robot interactions.33,34,35
Artificial Intelligence in Medicine
An exponential increase in the use of artificial intelligence in clinical settings has been seen in recent years. Artificial intelligence is used for medical tasks like diagnosis, prognosis, and therapy. The difference between traditional statistical analysis and artificial intelligence is that the latter utilises data-mining and pattern recognition capabilities to analyse structured and unstructured data.2
Structured data such as imaging, genetics, electrophysiological data are analysed using machine learning algorithms and unstructured data like electronic health records are evaluated using natural language processing techniques.24,36
From 2008 to 2015, the use of basic electronic health records systems among hospitals in the US has increased from 9.4% to 83.8 %.37 Analysis of electronic health records through artificial intelligence is supposed to enhance knowledge dissemination among clinicians and also empower patients to play an active role.36
Research on artificial intelligence in medicine, which was earlier limited to a few specialities like oncology, neurology, and cardiology, is at present encompassing every aspect of health care including surgical robots.24
Various fields of medicine influenced by artificial intelligence are briefly discussed in Table 2. 20,26,30,38-77
| Speciality | Role of Machine learning |
|---|---|
| Oncology | Detection and classification of lung cancers, skin lesions and breast cancer metastases26,38,39 Analyse the side effects of polypharmacy, radiotherapy, drug-drug and drug-protein interactions 40,41 Many CDSS, which are computer programs, are available that apply AI and provide clinicians/oncologist with evidence-based treatment recommendations for a variety of cancer diagnoses. Eg. CancerLinq, IBM’s WFO.42,43CDSS show varying degree of concordance with the treatment recommendations of the expert group with ovarian cancer showing highest concordance (96%)42,44 Newer frontiers of AI in oncology include predicting protein structure, classifying cells based on its cell cycle and drug development40,45,46 |
| Neurosurgery | Machine learning algorithms have been increasingly used for diagnosis, presurgical planning like tumour segmentation or epileptogenic zone localization, and outcome prediction47 Application in biomechanics and gait research48,49 |
| Radiology | AI is used in various radiological imaging tasks, such as risk assessment, detection, diagnosis, prognosis, and therapy response50 Machine learning models have been used in many aspects of radiology like detection of pulmonary lesions, fractures, organ laceration, mammography, malignancies and stroke.20The steps are similar to the image interpretation in dermatology given below |
| Drug development | Machine learning algorithms have been used in various stages of drug development, such as identification and validation of drug targets, designing of new drugs, drug repurposing, improving the R and D efficiency etc.51-53 AI can predict with high accuracy the likelihood of failure of a drug clinical trial, to determine the cancer cell sensitivity to therapeutics, or the peptide-major histocompatibility complex binding for oncologic immunotherapy development53-56 |
| Obstetrics | Machine learning algorithms are trained using features extracted from raw Cardiotocography foetal heart rate traces contained in the dataset to distinguish between caesarean section and vaginal delivery types57 Neural network model have been developed to detect epithelial ovarian cancer using noncoding RNAs and also used to improve the performance of assisted reproductive technology58,59 |
| Ophthalmology | Machine learning has been applied to ocular imaging like fundus photographs, optical coherence tomography and for ophthalmic diseases like diabetic retinopathy, glaucoma, age-related macular degeneration, retinopathy of prematurity and congenital cataracts60-62 |
| Cardiology | Machine learning models are able to identify different wave morphologies(QRS complexes, P and T waves) with high precision30 Machine learning models have also been used in cardiology for prediction of acute myocardial infarction by using proteomic measurements and clinical variables, estimation of in-stent restenosis from plasma metabolites, estimation of cardiovascular risks from electronic health records63-65 |
| Anaesthesiology | Machine learning in anaesthesiology has been studied to predict bispectral index using controlled infusion rates of propofol and remifentanil, to estimate hypotension using leverage data available during induction of anaesthesia and high-fidelity arterial line waveforms, to predict postoperative mortality from electronic health record data66-70 Robotics in anaesthesiology involved the introduction of McSleepy as a pharmacological robot to administer general anaesthesia using a closed loop system.71 SEDASYS® was the first computer-assisted personalized sedation system to receive US FDA approval in 2013. It was developed to allow mild-to-moderate propofol sedation to be delivered by non-anaesthesiologists for upper endoscopy and colonoscopy in healthy adults. It was discontinued from the market after 3 years72-74 |
| Psychiatry | Machine learning techniques have been used in psychiatry for the classification of dementia, attention deficit hyperactive disorder, schizophrenia75,76 Advancements in ML have also resulted in the creation of artificial intelligent agents in the form of highly realistic simulated psychotherapists, counsellors, and therapeutic coaches77 |
CDSS: Clinical decision-support computing system, AI: Artificial intelligence, WFO: Watson for Oncology, CAD: Computer-aided diagnosis, FDA: Food and Drug administration, MRI: Magnetic resonance imaging
Artificial intelligence in dermatology
Computer-based analysis of image assists in overcoming the subjective inter-observer and intra-observer variation thereby allowing an objective evaluation of parameters.78 There is an upward trend in the use of artificial intelligence as a diagnostic aid in dermatology. The application of computational methods is being used in dermatology for faster data processing to give better and more reliable diagnoses.79,80
This development began in the 1980s, when a greater public awareness was noted following an increased incidence of malignant melanoma, resulting in newer diagnostic modalities for early detection of lesions. Around the same time, Wilhelm Stolz and others in the Munich imaging group developed a hand-held dermatoscope for cutaneous surface microscopy.81 Later automated diagnosis of melanomas was tested using computer-aided diagnosis to reproduce the decision of the dermatologist when observing images of pigmented skin lesions either by photography, dermoscopy, and spectrophotometry.82
Several image processing software in the C programming language were written with the involvement of the dermatology digital imaging group. The focus was on automatic detection of lesional borders, malignant feature detection, detection of lesion changes, and algorithmic separation of benign from malignant lesions.81,82 In 1987, Cascinelli et al. studied the automated classification of pigmented skin lesions based on images.79,83 Over the last three decades, there is extensive literature on the use of machine learning models for analysing and classifying the data from skin lesions.84 It is also believed that an exponential expansion of smartphone technology worldwide will provide low-cost universal access to this vital diagnostic care.26
The general steps involved in computer-aided diagnosis include:
-
Image acquisition:
The two predominant dermatological image types are clinical and dermoscopic images with the increased use of the latter23
-
Image pre-processing: The term pre-processing in image diagnostic procedures usually encompasses artefact removal and lesion image enhancement.25 Good performance at this stage ensures the correct behaviour of the algorithms in the following stages of analysis.82
Artefact correction involves algorithms for removal of hair artefacts, dermoscopic gels, thin blood vessels, shadows, ruler markings, specular reflections and air bubbles, which can confuse diagnosis and impede the achievement of better accuracy in the automated diagnosis process.23,82,85 Out of these, hair shafts and ruler markings are the most commonly reported artefacts.23,85,86 Various machine learning models are used for this purpose. Filtering is a popular method to smooth a lesion image before detecting artefacts.23
Under image enhancement, the most important operation is colour calibration. This operation consists of recovering real colours of a photographed lesion, illumination correction, and contrast and edge enhancement.82 Commonly used colour spaces include red-green-blue and International Commission on Illumination among others.23,82
-
Image segmentation: Precise border detection (segmentation) is one of the most important and crucial areas in the automated analysis of pigmented skin lesions. It is also believed to be the most difficult task due to low-contrasts surrounding the skin, fuzzy borders, the existence of artefacts and irregular structures characterizing lesional images23,82
Various machine learning approaches have been described for the segmentation of lesions. They are based on the colour information of the lesion, luminance, and texture. They can be broadly classified as thresholding, edge-based, fuzzy c-means, gradient vector flow snakes or region-based methods. Thresholding method is the most commonly used one and achieves good results when there is good contrast between the lesion and the skin.23,82,87
-
Feature extraction: The extraction of specific features in a given lesion image is an essential step towards effective automated lesion image classification. The primary objective of feature extraction is to quantify the image by a set of finite numerical features.23 The various feature descriptors studied in the algorithm for melanoma analysis are based on dermoscopic (ABCD or pattern analysis) or clinical findings (ABCDE)88,89
A combination of photometric features like colour, islands of colour, colour homogeneity, colour histogram etc. and textural aspects yield good results in identifying pigmented skin lesions.23,90 The challenges in this process lie in the vast variety of images, body location, subject parameters (age), imaging parameters (lightening or camera), and the direction from which the lesion image is viewed.23
Classification of lesions: Lesion classification is the last step in the process for the computerized analysis of images. Image classification involves using selected features of an image to classify pixels of the image into one of the several classes depending on specific knowledge domain.
The two main classification types as reported in the literature in relation to medical imaging are supervised classification and unsupervised classification (already discussed). The literature has reported the application of several classification methods for lesion images. Frequently used among these methods are the artificial neural network, support vector machine, decision trees, k-nearest neighbour, regression analysis classifiers, bayesian classifiers and fuzzy logic.20,21,23,82
Artificial Intelligence Applications in Dermatological Conditions
Automated detection of skin lesion using images has extended beyond melanoma to encompass pigmentary skin lesions, non-melanocytic skin cancers, psoriasis, skin rash, and onychomycosis among other skin diseases.26, 91-95
Pigmentary skin lesions and malignancy
Many research articles have been published classifying nonmelanoma skin cancers vs. benign and pre-malignant lesions with varying efficacy between different artificial intelligence systems.96,97 Many systems are commercially available for computer-aided diagnosis of pigmented skin lesions which are mainly based on dermoscopy. e.g. DANAOS expert systems, DBDermo-Mips, MoleAnalyser expert systems.82 Multiple smartphone based apps like SkinVision, DermaAId, Skin 10, MoleScope are available for screening skin cancers, tracking changes in moles on the skin and identification of basic skin lesions.98 Studies done on the SkinVision app scored an 80% sensitivity and 78% specificity in detecting premalignant conditions.99
Psoriasis
Artificial intelligence has been working on many aspects of psoriasis. Various computer aided diagnostic systems have been designed for image classification and psoriasis risk stratification.91,100 Also machine learning prediction models have been designed to determine the treatment response of psoriasis to biologics and to differentiate psoriasis from psoriatic arthritis using genetic markers.101,102 Correa da Rosa et al. showed that the gene-expression profiles of psoriasis skin lesions, taken in the first 4 weeks on patients who are on treatment with a biological agent, can be used to accurately predict (>80% area under the ROC curve) the clinical endpoint at 12 weeks using machine learning techniques thereby reducing the assessment gap by 2 months.103
Emam et al. studied whether machine learning could aid in predicting long-term responses to biologics in psoriasis through analysis of data of 681 psoriasis patients from the Danish registry cohort using various modelling techniques. Patients with early diagnosis and early initiation of treatment, without psoriatic arthritis, had 90% chance of continuing treatment as per the study.104 Foulkes et al. noted that signals of response to therapy in patients with severe psoriasis treated with the etanercept may be systemically detectable in lesional skin, non lesional skin, and blood at baseline, before the commencement of therapy.105 Automated diagnosis of other erythemato-squamous diseases such as seborrheic dermatitis, atopic dermatitis, lichen planus, pityriasis rosea and pityriasis rubra pilaris has been studied using various clinical and histopathological features.106,107
Acne
New technologies in imaging and software solutions have been developed in acne and rosacea evaluation. A study by Min et al. showed that compared with manual counting performed by an expert dermatologist, the sensitivity and positive predictive value of the lesion-counting program was greater than 70% for papules, nodules, pustules, and whitehead comedones.108,109
Autoimmune disorders
Machine learning has been used in various aspects of patient identification, risk prediction, diagnosis, disease subtype classification, disease progression and outcome and monitoring and management of autoimmune disorders like systemic lupus erythematosus, systemic sclerosis, vitiligo, psoriatic arthritis, rheumatoid arthritis, and systemic vasculitis.110
Also, machine learning techniques have been used for automated or semi-automated classification of myositis using ultrasound images. The study differentiated between normal muscle, dermatomyositis, polymyositis, and inclusion body myositis with accuracies above 70%.97,111
Allergic contact dermatitis
Contact dermatitis is a common immunological reaction to sensitizers. The predictive screenings for the sensitizing chemicals have been usually performed using animal models such as the murine local lymph node assay. Animal models for sensitization assessment are being replaced by non-animal testing methods like Genomic Allergen Rapid Detection assay, which is based on measurements of transcriptional levels of genomic biomarkers. Artificial intelligence algorithms were used to study the changes induced in these genomic biomarkers as compared to profiles of reference chemicals on cellular stimulation with unknown sensitizers.112,113
Ulcer evaluation and prediction
The common risk factors for chronic wounds in India are diabetes, tuberculosis, leprosy, vascular disorders, pressure ulcer, and trauma. Automated analysis has been used in various aspects of ulcer assessment like analysis of wound perimeter, surface, depth, area determination, wound delineation and composition thereby providing an objective and quantitative assessment of healing rate during treatment.114-117 Artificial intelligence applications are also used in predicting the development of pressure injuries among surgical critical care patients.118
Robotics
Robots represent the physical aspect of artificial intelligence. In healthcare, Robot-assisted biopsies were one of the first uses of surgical robots and have been used for prostate, lung, breast and stereotactic brain biopsies.119-122 Automated device for performing skin biopsies has been developed by scientists. Wherein the biopsy can be performed with fewer instruments within a shorter duration of time without the necessity of local anaesthesia.123,124 Robot-assisted automatic laser hair removal system has been developed to automatically detect any arbitrary shape of the desired treatment area and to provide uniform laser irradiation to the designated skin area.125 Studies on the laser hair removal system system provides consistent irradiation not only in terms of uniform distance and also the number of irradiation shots.126 A robotic system for hair restoration (the ARTAS system) has been developed for the follicular unit extraction type of hair restoration surgery. It consists of a robotic system with a stereoscopic camera with high resolution to identify hair follicles for harvesting and implantation.127,128
Human versus Artificial Intelligence
Human interpretation of images may be limited by the presence of structure noise, incomplete visual search patterns, fatigue, distractions, vast amounts of image data, and the physical quality of the image itself.50 With the evolution of machine learning techniques, the question among artificial intelligence experts is when, rather than whether, artificial intelligence models will outsmart their human counterparts. They believe in the next decade that artificial intelligence will outperform humans in many activities such as translation of languages and driving a vehicle. Also, in another 50 years, artificial intelligence may surpass the brainpower equivalent to that of all human beings combined i.e. attain singularity and probably be writing bestsellers, doing maths research and even perform surgeries.4,129-131 Time will tell whether these predictions will fructify.
At present, in dermatology, many studies using varied algorithms have shown results ranging from equal or better efficacy. Comparison of human and deep learning systems for the management of small pigmentary skin lesions have demonstrated that for small lesions, the computer-vision system had significantly higher sensitivity and in a few studies outperformed dermoscopists.130,132 A study by Esteva et al. analysed clinical and dermoscopic images using a single convolutional neural network, trained from images directly, using only pixels and disease labels as inputs. This is unlike other techniques which required extensive pre-processing, lesion segmentation and extraction of specific features before classification. The convolutional neural network was trained using a dataset of 129,450 clinical images consisting of 2032 different diseases. Its performance was tested against board-certified dermatologists on biopsy-proven clinical images to differentiate between benign pigmentary skin lesions and melanoma. The convolutional neural network demonstrated performance on par with tested experts across both tasks.26 MacLellan et al. analysed artificial intelligence in the diagnosis of melanomas in clinical settings, which was not done in many of the previous studies and found an increased sensitivity of the local dermatologist (96.6%) as compared to the computer-based algorithm (88.1%). The study concluded that these tools would aid in better diagnosis but will not replace clinical decision making.133
Deep learning algorithms achieved better diagnostic performance than pathologists in identifying malignant tumours and detecting lymph node metastases in tissue sections of women with breast cancer.4,39 In the field of radiology too, artificial intelligence systems have been on par with, and sometimes outperformed radiologists. Rajpurkar et al. found that deep learning models detected clinically important abnormalities (e.g., oedema, fibrosis, mass, pneumonia, and pneumothorax) on chest radiography, at a performance level comparable to practicing radiologists.39,131,134,135
Artificial Intelligence Limitations
Data
Artificial intelligence applications are only as robust as the data on which they are trained. Deep learning neural networks require large amounts of data. This can be a drawback when artificial intelligence is attempted on a disease with low prevalence or when data is generalised across different populations. The heterogeneity of medical data across institutions and the complexity of the neural networks can lead to over fitting models.4,37,40 In addition, the quality of the information extracted, is still dependent on the accuracy of the input data being entered and the infrastructure available for data sharing. Also, algorithms can underperform in conditions where there is no precedence like new side effects of drugs or treatment resistance.1,40,
Acceptance
Detailed history and thorough clinical examination complemented by relevant investigations is the basis of diagnosis in most dermatoses. Histopathology still remains the gold standard diagnostic investigation as compared to other noninvasive investigations including artificial intelligence. Also patient care is not restricted to diagnosis but requires a holistic approach with human touch which cannot be replaced by algorithm. All these factors may challenge the acceptance of artificial intelligence in dermatology.136,137
Reasoning (the black box problem)
In medical practice, it has traditionally been essential in clinical decision-making to know the rationale for each decision. In contrast, DL utilizes unstructured input data, and the bulk of output generation occurs within the hidden layers. It thus becomes difficult to determine which specific feature or calculation of the input data contributed to the resultant output.40,137
Legal liability and ethics
The efficacy of the algorithms depends on large data and certain data may infringe on patients’ privacy. Therefore, it is important to create standard ethical guidelines wherein artificial intelligence can be applied and where they are mandatory. Also, in the case of an adverse event, accountability is still a grey area that has to be addressed.4,137,138
Bias
Algorithms may also inherit the bias of the programmer or the self-learning algorithms may learn to be biased due to lack of diversity in the training material. As most machine learning programs are based on patient data which are collected from fair skinned populations, they may underperform on images of lesions in the skin of colour.4,13,139
Job threat
There is a huge debate among experts whether or not artificial intelligence will result in large scale job losses. Some believe that Artificial Intelligence is set to take over 47% of the U.S. employment market within 20 years across all sections.140,141 Since medical imaging is an important field associated with artificial intelligence, some experts fear a job threat for these sub-specialities, while others believe that there is no immediate threat of job loss, but working with artificial intelligence systems will enhance diagnostic efficacy and benefit patient care.131,142,143
Limitations of the article
The article focuses on a basic understanding of artificial intelligence in healthcare and its applications in dermatology and is limited by the lack of in-depth computer knowledge of the authors. Furthermore, the applications and implications of artificial intelligence in healthcare are vast and ever-expanding. Therefore, these cannot be entirely covered in such a brief review.
Conclusion
Any new technology has its inherent advantages and disadvantages. The success of such technology in dermatology will depend on the benefits it provides to the vast majority of the general population and also to the treating doctor. Though artificial intelligence technology is not intended to replace medical professionals, their role is going to undergo change in the era of artificial intelligence. The limitations of applications of artificial intelligence in dermatology are very stark, but considering the rapid increase in efficiency of artificial intelligence models on one hand, and the ever demanding workload of doctors, the integration of artificial intelligence into healthcare may become the essential feature of medical care in the future. Medical personnel will then be able to give more focus on human emotional components like care and compassion with renewed vigour, which are important aspects of the doctor-patient relationship in medical care.
Acknowledgement
Authors would like to acknowledge the help of Mr Arvind Shenoy, principal consultant, Infosys for the technical inputs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The role of artificial intelligence in precision medicine. Expert Rev Precision Med Drug Dev. 2017;2:239-41.
- [CrossRef] [Google Scholar]
- Use of Artificial Intelligence in Healthcare Delivery, eHealth-Making Health Care Smarter, Heston TF. 2018. IntechOpen. Available from: https://www.intechopen.com/books/ehealth-making-health-care-smarter/use-of-artificial-intelligence-in-healthcare-delivery [Last accessed on 2020 Apr 30]
- [CrossRef] [Google Scholar]
- Artificial Intelligence. 2019. The Free Encyclopedia. Available from: https://en.wikipedia.org/w/index.php?title=Artificial_intelligence&oldid=902861480 [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Medicine and the rise of the robots: A qualitative review of recent advances of artificial intelligence in health. BMJ Leader. 2018;2:59-63.
- [CrossRef] [Google Scholar]
- Artificial intelligence in medicine. Metabolism. 2017;69S:S36-40.
- [CrossRef] [PubMed] [Google Scholar]
- History of Artificial Intelligence. 2020. Wikipedia, The Free Encyclopedia. Available from: https://en.wikipedia.org/w/index.php?title=History_of_artificial_intelligence&oldid=952036041 [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-8.
- [CrossRef] [PubMed] [Google Scholar]
- 2019. The Stanford Encyclopedia of Philosophy. Available from https://plato.stanford.edu/archives/spr2019/entries/turing-test/ [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Mastering the game of Go without human knowledge. Nature. 2017;550:354-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mastering chess and shogi by self-play with a general reinforcement learning algorithm In: arXiv. 171201815v1; 2017
- [Google Scholar]
- Global evolution of research in artificial intelligence in health and medicine: A Bibliometric Study. J Clin Med. 2019;8:E360.
- [CrossRef] [PubMed] [Google Scholar]
- The Cambridge Handbook of Artificial Intelligence Cambridge, UK: Cambridge University Press; 2014. p. :1-14.
- [CrossRef] [Google Scholar]
- Your Future Doctor May Not be Human. 2018. This Is the Rise of AI in Medicine. Available from: https://futurism.com/ai-medicine-doctor [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Artificial Intelligence in Law: The State of Play 2016. 2016. Available from: https://www.neotalogic.com/wp-content/uploads/2016/04/Artificial-Intelligence-in-Law-The-State-of-Play-2016.pdf [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Top 10 Hot Artificial Intelligence (AI) Technologies. 2017. Available from: https://www.forbes.com/sites/gilpress/2017/01/23/top-10-hot-artificial-intelligence-ai-technologies/#445d61c31928 [Last accessed on 2020 Apr 30]
- [Google Scholar]
- The Difference between Artificial Intelligence. 2017. Machine Learning, and Deep Learning. Available from: https://medium.com/iotforall/the-difference-between-artificial-intelligence-machine-learning-and-deep-learning-3aa67bff5991 [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Natural and Artificial intelligence in neurosurgery: A systematic review. Neurosurgery. 2018;83:181-92.
- [CrossRef] [PubMed] [Google Scholar]
- How artificial intelligence, machine learning and deep learning are radically different? Int J Adv Res Comput Sci Software Eng. 2018;8:2277-128.
- [CrossRef] [Google Scholar]
- A systematic review on machine learning in neurosurgery: The future of decision-making in patient care. Turk Neurosurg. 2018;28:167-73.
- [CrossRef] [PubMed] [Google Scholar]
- Current applications and future impact of machine learning in radiology. Radiology. 2018;288:318-28.
- [CrossRef] [PubMed] [Google Scholar]
- Implementing machine learning in radiology practice and research. AJR Am J Roentgenol. 2017;208:754-60.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in cardiology. J Am Coll Cardiol. 2018;71:2668-79.
- [CrossRef] [PubMed] [Google Scholar]
- A review of prevalent methods for automatic skin lesion diagnosis. Open Dermatol J. 2018;12:14-53.
- [CrossRef] [Google Scholar]
- Artificial intelligence in healthcare: Past, present and future. Stroke Vasc Neurol. 2017;2:230-43.
- [CrossRef] [PubMed] [Google Scholar]
- A Beginner's Guide to Neural Networks and Deep Learning. Available from: https://pathmind.com/wiki/neural-network [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Dermatologist-level classification of skin Cancer with deep neural networks. Nature. 2017;542:115-8.
- [CrossRef] [PubMed] [Google Scholar]
- Convolutional neural networks: an overview and application in radiology. Insights Imaging. 2018;9:611-629.
- [CrossRef] [PubMed] [Google Scholar]
- Tutorial on Support Vector Machine (SVM) Available from: https://course.ccs.neu.edu/cs5100f11/resources/jakkula.pdf [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Overview of advanced computer vision systems for skin lesions characterization. IEEE Trans Inf Technol Biomed. 2009;13:721-33.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. 2019;40:1975-86.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-48.
- [CrossRef] [PubMed] [Google Scholar]
- An introduction to computer vision in medical imaging In: Chen CH editor. Computer Vision in Medical Imaging. Singapore: World Scientific Publishing; 2014. p. :1-17.
- [CrossRef] [PubMed] [Google Scholar]
- Image analysis and computer vision in medicine. Computerized Medical Imaging and Graphics. 1994;18:85-96.
- [CrossRef] [Google Scholar]
- The inevitable application of big data to health care. JAMA. 2013;309:1351-2.
- [CrossRef] [PubMed] [Google Scholar]
- Adoption of Electronic Health Record Systems among U.S. Non-Federal Acute Care Hospitals: 2008-2015. 2016. Available from: https://dashboard.healthit.gov/evaluations/data-briefs/non-federal-acute-care-hospital-ehr-adoption-2008-2015.php [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24:1559-67.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318:2199-210.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in oncology: Current applications and future directions. Oncology (Williston Park). 2019;33:46-53.
- [Google Scholar]
- Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics. 2018;34:i457-i466.
- [CrossRef] [PubMed] [Google Scholar]
- Watson for oncology and breast cancer treatment recommendations: Agreement with an expert multidisciplinary tumor board. Ann Oncol. 2018;29:418-23.
- [CrossRef] [PubMed] [Google Scholar]
- Qualitative Study of Oncologists' Views on the CancerLinQ Rapid Learning System. J Oncol Pract. 2017;13:e176-e184.
- [CrossRef] [PubMed] [Google Scholar]
- Concordance study between IBM Watson for oncology and clinical practice for patients with cancer in China. Oncologist. 2019;24:812-9.
- [CrossRef] [PubMed] [Google Scholar]
- Computational protein design with deep learning neural networks. Sci Rep. 2018;8:6349.
- [CrossRef] [PubMed] [Google Scholar]
- Reconstructing cell cycle and disease progression using deep learning. Nat Commun. 2017;8:463.
- [CrossRef] [PubMed] [Google Scholar]
- Natural and artificial intelligence in neurosurgery: A systematic review. Neurosurgery. 2017;83:181-192.
- [CrossRef] [PubMed] [Google Scholar]
- Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247-50.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning algorithms based on signals from a single wearable inertial sensor can detect surface-and age-related differences in walking. J Biomech. 2018;71:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning in medical imaging. J Am Coll Radiol. 2018;15:512-20.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in drug development: Present status and future prospects. Drug Discov Today. 2019;24:773-80.
- [CrossRef] [PubMed] [Google Scholar]
- Applications of deep learning in biomedicine. Mol Pharm. 2016;13:1445-54.
- [CrossRef] [PubMed] [Google Scholar]
- How artificial intelligence is changing drug discovery. Nature. 2018;557:S55-S57.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning prediction of cancer cell sensitivity to drugs based on genomic and chemical properties. PLoS One. 2013;8:e61318.
- [CrossRef] [PubMed] [Google Scholar]
- Letter: Change in trypsin sensitivity during structural rearrangements in biological membranes. Biofizika. 1975;20:942-4.
- [Google Scholar]
- Deep convolutional neural networks for pan-specific peptide-MHC class I binding prediction. BMC Bioinformatics. 2017;18:585.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning ensemble modelling to classify caesarean section and vaginal delivery types using Cardiotocography traces. Comput Biol Med. 2018;93:7-16.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence: The future of obstetrics and gynecology. J Obstet Gynaecol India. 2018;68:326-7.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in reproductive medicine. Reproduction. 2019;158:R139-R154.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103:167-75.
- [CrossRef] [PubMed] [Google Scholar]
- An artificial intelligence platform for the multihospital collaborative management of congenital cataracts. Nature Biomed Eng. 2017;1:24.
- [CrossRef] [Google Scholar]
- Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402-10.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of a prediction model for the development of atrial fibrillation in a repository of electronic medical records. JAMA Cardiol. 2016;1:1007-13.
- [CrossRef] [PubMed] [Google Scholar]
- Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68:2287-95.
- [CrossRef] [PubMed] [Google Scholar]
- Using recurrent neural network models for early detection of heart failure onset. J Am Med Inform Assoc. 2017;24:361-70.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of Bispectral Index during Target-controlled Infusion of Propofol and Remifentanil: A deep learning approach. Anesthesiology. 2018;128:492-501.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence for anesthesia: What the practicing clinician needs to know: More than black magic for the art of the dark. Anesthesiology. 2018;129:619-22.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a deep neural network model for prediction of postoperative in-hospital mortality. Anesthesiology. 2018;129:649-62.
- [CrossRef] [PubMed] [Google Scholar]
- Supervised machine-learning predictive analytics for prediction of postinduction hypotension. Anesthesiology. 2018;129:675-88.
- [CrossRef] [PubMed] [Google Scholar]
- Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology. 2018;129:663-74.
- [CrossRef] [PubMed] [Google Scholar]
- SEDASYS System. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/P080009c.pdf [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Monitoring and delivery of sedation. Br J Anaesth. 2014;113(Suppl 2):ii37-47.
- [CrossRef] [PubMed] [Google Scholar]
- Johnson & Johnson To Stop Selling Sedasys System. 2016. Available from: https://www.anesthesiologynews.com/Web-Only/Article/03-16/Johnson-amp-Johnson-To-Stop-Selling-Sedasys-System/35650 [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Deep neural networks in psychiatry. Mol Psychiatry. 2019;24:1583-98.
- [CrossRef] [PubMed] [Google Scholar]
- Towards artificial intelligence in mental health by improving schizophrenia prediction with multiple brain parcellation ensemble-learning. NPJ Schizophr. 2019;5:2.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for the ethical use and design of artificial intelligent care providers. Artif Intell Med. 2014;62:1-0.
- [CrossRef] [PubMed] [Google Scholar]
- Computer aided diagnostic support system for skin cancer: A review of techniques and algorithms. Int J Biomed Imaging. 2013;2013:323268.
- [CrossRef] [PubMed] [Google Scholar]
- Automated detection of nonmelanoma skin cancer using digital images: A systematic review. BMC Med Imaging. 2019;19:21.
- [CrossRef] [PubMed] [Google Scholar]
- Digital imaging in dermatology. Comput Med Imaging Graph. 1992;16:145-50.
- [CrossRef] [Google Scholar]
- Computerized analysis of pigmented skin lesions: A review. Artif Intell Med. 2012;56:69-90.
- [CrossRef] [PubMed] [Google Scholar]
- A possible new tool for clinical diagnosis of melanoma: The computer. J Am Acad Dermatol. 1987;16:361-7.
- [CrossRef] [Google Scholar]
- Automated diagnostic instruments for cutaneous melanoma. Semin Cutan Med Surg. 2008;27:32-6.
- [CrossRef] [PubMed] [Google Scholar]
- E-shaver: an improved DullRazor(®) for digitally removing dark and light-colored hairs in dermoscopic images. Comput Biol Med. 2011;41:139-45.
- [CrossRef] [PubMed] [Google Scholar]
- Computational methods for the image segmentation of pigmented skin lesions: A review. Comput Methods Programs Biomed. 2016;131:127-41.
- [CrossRef] [PubMed] [Google Scholar]
- Pattern analysis of dermoscopic images based on Markov random fields. Pattern Recognit. 2009;42:1052-7.
- [CrossRef] [Google Scholar]
- Dermoscopy of pigmented skin lesions. J Am Acad Dermatol. 2005;52:109-21.
- [CrossRef] [PubMed] [Google Scholar]
- Dermoscopy report: proposal for standardization. Results of a consensus meeting of the International Dermoscopy Society. J Am Acad Dermatol. 2007;57:84-95.
- [CrossRef] [PubMed] [Google Scholar]
- Two systems for the detection of melanomas in dermoscopy images using texture and color features. IEEE Systems Journal. 2014;8:965-979.
- [CrossRef] [Google Scholar]
- Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: A first comparative study of its kind. Comput Methods Programs Biomed. 2016;126:98-109.
- [CrossRef] [PubMed] [Google Scholar]
- Cutting edge technology in dermatology: Virtual reality and artificial intelligence. Cutis;. 2018;101:236-237.
- [Google Scholar]
- Automatic psoriasis lesion segmentation in two-dimensional skin images using multiscale superpixel clustering. J Med Imaging (Bellingham). 2017;4:44004.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence based skin classification using GMM. J Med Syst. 2018;43:3.
- [CrossRef] [PubMed] [Google Scholar]
- Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: Automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13:e0191493.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Invest Dermatol. 2018;138:1529-38.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence applications in dermatology: Where do we stand? Front Med (Lausanne). 2020;7:100.
- [CrossRef] [PubMed] [Google Scholar]
- Machine Learning for Dermatology - 5 Current Applications. 2019. Available from: http://emerj.com/ai-sector-overview/machine-learning-dermatology-applications [Last accessed on 2020 Apr 30]
- [Google Scholar]
- mHealth app for risk assessment of pigmented and non pigmented skin lesions-A study on sensitivity and specificity in detecting malignancy. Telemed J E Health. 2017;23:948-54.
- [CrossRef] [PubMed] [Google Scholar]
- A novel and robust Bayesian approach for segmentation of psoriasis lesions and its risk stratification. Comput Methods Programs Biomed. 2017;150:9-22.
- [CrossRef] [PubMed] [Google Scholar]
- Machine learning in dermatology: Current applications, opportunities, and limitations. Dermatol Ther. 2020;10:365-386.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic signature to provide robust risk assessment of psoriatic arthritis development in psoriasis patients. Nat Commun. 2018;9:4178.
- [CrossRef] [PubMed] [Google Scholar]
- Shrinking the psoriasis assessment gap: Early gene-expression profiling accurately predicts response to long-term treatment. J Invest Dermatol. 2017;137:305-12.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting the long-term outcomes of biologics in patients with psoriasis using machine learning. Br J Dermatol. 2020;182:1305-1307.
- [CrossRef] [PubMed] [Google Scholar]
- A framework for multi-omic prediction of treatment response to biologic therapy for psoriasis. J Invest Dermatol. 2019;139:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- Automatic detection of erythemato-squamous diseases using adaptive neuro-fuzzy inference systems. Comput Biol Med. 2005;35:421-33.
- [CrossRef] [PubMed] [Google Scholar]
- Design and evaluation of a multi-model, multi-level artificial neural network for eczema skin lesion detection In: 2015 3rd International Conference on Artificial Intelligence, Modelling and Simulation (AIMS). 2015. p. :42-7. Available from: https://ieeexplore.ieee.org/document/7604549 [Last accessed on 2020 Apr 30]
- [CrossRef] [Google Scholar]
- Development and evaluation of an automatic acne lesion detection program using digital image processing. Skin Res Technol. 2013;19:e423-32.
- [CrossRef] [PubMed] [Google Scholar]
- Ros-NET: A deep convolutional neural network for automatic identification of rosacea lesions. Skin Res Technol. 2020;26:413-421.
- [CrossRef] [PubMed] [Google Scholar]
- A systematic review of the applications of artificial intelligence and machine learning in autoimmune diseases. NPJ Digital Med. 2020;3:30.
- [CrossRef] [PubMed] [Google Scholar]
- Automated diagnosis of myositis from muscle ultrasound: exploring the use of machine learning and deep learning methods. PLoS One. 2017;12:e0184059.
- [CrossRef] [PubMed] [Google Scholar]
- From genome-wide arrays to tailor-made biomarker readout-Progress towards routine analysis of skin sensitizing chemicals with GARD. Toxicol In Vitro. 2016;37:178-88.
- [CrossRef] [PubMed] [Google Scholar]
- Local lymph node assay-Validation, conduct and use in practice. Food Chem Toxicol. 2002;40:593-8.
- [CrossRef] [Google Scholar]
- Towards a comprehensive assessment of wound-composition using color-image processing. In Proceedings of the IEEE International Conference on Image Processing (ICIP '09); November 2009:4185-8.
- [CrossRef] [Google Scholar]
- Fuzzy spectral clustering for automated delineation of chronic wound region using digital images. Comput Biol Med. 2017;89:551-60.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of pressure ulcer tissues with 3D convolutional neural network. Med Biol Eng Comput. 2018;56:2245-58.
- [CrossRef] [PubMed] [Google Scholar]
- Automated tissue classification framework for reproducible chronic wound assessment. BioMed Res Int. 2014;2014:851582.
- [CrossRef] [PubMed] [Google Scholar]
- Predicting pressure injury in critical care patients: A machine-learning model. Am J Crit Care. 2018;27:461-8.
- [CrossRef] [PubMed] [Google Scholar]
- Robot-assisted stereotactic brain biopsy: Systematic review and bibliometric analysis. Childs Nerv Syst. 2018;34:1299-309.
- [CrossRef] [PubMed] [Google Scholar]
- Global trends in paediatric robot-assisted urological surgery: A bibliometric and Progressive Scholarly Acceptance analysis. J Robot Surg. 2018;12:109-15.
- [CrossRef] [PubMed] [Google Scholar]
- Stormram 4: An MR safe robotic system for breast biopsy. Ann Biomed Eng. 2018;46:1686-96.
- [CrossRef] [PubMed] [Google Scholar]
- Ion by Intuitive. Available from: https://www.intuitive.com/en-us/products-and-services/ion [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Automatic Device for Skin Biopsy: Advances in Theory and Practice. In New Trends in Medical and Service Robotics: Advances in Theory and Practice 2019:54-61.
- [CrossRef] [Google Scholar]
- Universidad Carlos III de Madrid - Oficina de Información Científica. 2015. Available from: https://www.sciencedaily.com/releases/2015/05/150507082445.htm [Last accessed on 2020 Apr 30]
- [Google Scholar]
- A study on the development of a robot-assisted automatic laser hair removal system. Photomed Laser Surg. 2014;32:633-41.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of efficacy between novel robot-assisted laser hair removal and physician-directed hair removal. Photomed Laser Surg. 2015;33:509-16.
- [CrossRef] [PubMed] [Google Scholar]
- Artas. Available from: https://artas.com/about-the-artas-procedure [Last accessed on 2020 Apr 30]
- [Google Scholar]
- When will AI exceed human performance? Evidence from AI experts. 2017;arXiv:1705.08807.
- [CrossRef] [Google Scholar]
- The diagnostic performance of expert dermoscopists vs. a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-82.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in radiology: Friend or foe? Where are we now and where are we heading? Acta Radiol Open. 2019;8:2058460119830222.
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning outperformed 136 of 157 dermatologist in a head to head dermoscopic melanoma image classification task. Eur J Cancer. 2019;113:47-54.
- [CrossRef] [PubMed] [Google Scholar]
- The use of non-invasive imaging techniques in the diagnosis of melanoma: A prospective diagnostic accuracy study. J Am Acad Dermatol 2020:S0190-9622(20)30559-4.
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning for chest radiograph diagnosis: A retrospective comparison of CheXNeXt to practicing radiologists. PLoS Med. 2018;15:e1002686.
- [CrossRef] [PubMed] [Google Scholar]
- Deep learning and artificial intelligence in radiology: Current applications and future directions. PLoS Med. 2018;15:e1002707.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial intelligence in dermatology: are we there yet? Br J Dermatol. 2019;181:190-191.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial Intelligence Applications in Dermatology: Where Do We Stand? Front Med (Lausanne). 2020;7:100.
- [CrossRef] [PubMed] [Google Scholar]
- Artificial Intelligence. 2017. Medical Malpractice, and the End of Defensive Medicine. Available from: http://blog.petrieflom.law.harvard.edu/2017/01/26/artificial-intelligence-medical-malpractice-and-the-end-of-defensive-medicine/ [Last accessed on 2020 Apr 30]
- [Google Scholar]
- Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247-8.
- [CrossRef] [PubMed] [Google Scholar]
- The future of employment: How susceptible are jobs to computerisation? Available from: https://www.oxfordmartin.ox.ac.uk/publications/the-future-of-employment/[Last accessed on 2020 Apr 30]
- [Google Scholar]
- Artificial Intelligence in Healthcare: Separating Reality from Hype. 2018. Available form: https://www.forbes.com/sites/robertpearl/2018/03/13/artificial-intelligence-inhealthcare/#43b3ec271d75 [Last accessed on 2020 Apr 30]
- [Google Scholar]
- AI Will Lead To Job Losses in NHS Radiology Departments. 2018. Available form: https://ai-med.io/ai-job-losses-nhs-radiology/ [Last accessed on 2020 Apr 30]
- [Google Scholar]
- The end of radiology? Three threats to the future practice of radiology. J Am Coll Radiol. 2016;13:1415-20.
- [CrossRef] [PubMed] [Google Scholar]






