Translate this page into:
Epilepsy and syphilis: A systematic review and meta-analysis
Corresponding author: Dr. Wei Yue, Department of Neurology, Tianjin Huanhu Hospital, 6 Jizhao Rd., Tianjin, 300350, China. hhyuewei2008@163.com
-
Received: ,
Accepted: ,
How to cite this article: Zhang C, Yue W, Hou S, Cui W, Xiang L. Epilepsy and syphilis: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol 2021;87:483-90.
Abstract
Background:
Epileptic seizures were noted as one of the most overlooked manifestations in syphilis; therefore a few clinicians are concerned about the relationship between epilepsy and syphilis. Our study sought to clarify the prevalence and clinical features of epileptic seizures in patients with syphilis.
Methods:
We retrieved relevant articles from different databases, using the keywords “syphilis and epilepsy” and then performed statistical analysis to characterize the relationship between these diseases.
Results:
Forty one articles were included in this study: eight described the prevalence of syphilis and epilepsy and the remaining 33 were case reports on syphilis with epileptic seizures. The meta-analysis included 1252 patients with syphilis. The pooled estimate of proportion of prevalence (95% confidence interval) was 0.1384 (0.0955–0.2005), and the proportion and heterogeneity showed different degrees of change among three subgroups. The systematic review included 46 cases of syphilis with epileptic seizures. Thirty two (80%) patients had motor seizures, among whom 20 (62.5%) had tonic-clonic seizures. In addition, 30 (75%) patients had impaired awareness and 18 (45%) had status seizures. Twenty five (62.5%) patients were 35–55 years of age, and 77.5% of the included patients were men. Thirty seven (97.4%) patients were seizure-free after anti-syphilis treatment.
Limitations:
Research in this field has been conducted for a relatively short period and publication bias may exist. Furthermore, some patients with syphilis and epileptic seizures may not have received a clear diagnosis.
Conclusion:
The proportion of prevalence was 0.1384. Most of the included patients were 35–55 years of age and had impaired awareness and motor seizures. Many patients with syphilis and epileptic seizure showed full recovery or the development of minor neurological sequelae, and nearly all patients were seizure-free after timely anti-syphilis treatment.
Keywords
Clinical features
epilepsy
meta-analysis
prevalence
syphilis
Plain Language Summary
Syphilis is one of the most neglected pathogenic factors related to epileptic seizures. This study aimed to clarify the prevalence of epileptic seizures due to syphilis and examine the clinical features by performing a meta-analysis and literature review. The authors searched for articles using the key words “syphilis and epilepsy” in the different databases. After the exclusion of duplicate and irrelevant studies, 49 articles were utilised for further analyses. The results suggested that the proportion of prevalence of epileptic seizures among patients with syphilis was 0.1384 in a random-effect model. The cases with seizure and syphilis were observed in 35–55 years of age. The most common seizure presentations are impaired awareness and motor seizures.
Introduction
Epilepsy affects approximately 65 million people worldwide and is one of the most common neurological disorders.1,2 Notably, damage to the brain due to epilepsy may have many undesirable consequences. Traditional treatment with antiepileptic drugs or the ketogenic diet can reduce the risk of recurrence within the first few years after diagnosis but may not enhance the quality of life in affected patients; epilepsy remains uncontrolled in more than one-third of patients.3,4 Identification of the cause of epilepsy and limitation of the disease’s further relapses is important to avoid potential further neuronal damage.5,6
Syphilis is one of the most neglected pathogenic factors related to epileptic seizures and can lead to serious consequences.7 In recent years, with the indiscriminate use of antibiotics, the symptoms of syphilis have been considerably modified, often leading to atypical or masked forms.8 Neurosyphilis has been proved to develop in the primary stage also, although classically it occurs in the tertiary stage of syphilis, and epilepsy has often been reported as the lone and initial manifestation of neurosyphilis.9,10 Briefly, the incidence of syphilis has increased in recent years and has exhibited a modification of its salient presentations; it is a challenge for clinicians to distinguish epileptic seizures caused by syphilis, especially in patients with no typical symptoms of syphilis.10-12 Our study aims to clarify the prevalence of epileptic seizures due to syphilis and examine the clinical features by performing a meta-analysis and literature review.
Methods
Literature search
To define the relationship between syphilis and epilepsy, we searched for articles published through June 1, 2018, using the keywords “syphilis and epilepsy” in the following databases: PubMed, Cochrane Library, Embase, WHOLIS, SciELO, Clinicaltrails.gov and Web of Science. To ensure that search results were comprehensive and consistent, two authors performed the same search independently.
Selection criteria
Epilepsy prevalence among patients with syphilis: To enable the identification of patients with syphilis and epileptic seizures, the selected studies were required to include all patients with syphilis in a certain area and period of time. We also required patients to be divided into groups based on symptoms. We required disease diagnoses to be made in accordance with the diagnostic criteria of the time, and articles to be published in English within the past 50 years (1968–2018). When the two authors disagreed on the inclusion of an article, a third author judged the article’s eligibility for inclusion.
Case reports of epileptic seizures in patients with syphilis: to perform a comprehensive systematic review of epilepsy and syphilis, we searched all articles describing epilepsy and syphilis. Basic characteristics of the patients were required for further analysis, as well as a brief description of the main symptoms to define the type of epileptic seizure. In addition, we required a clear diagnosis of syphilis, such as at least one positive reaction from blood or cerebrospinal fluid; either the Venereal Disease Research Laboratory test, rapid plasma reagin test, toluidine red unheated serum test, Treponema pallidum particle agglutination test, Treponema pallidum hemagglutination assay, fluorescent treponemal antibody absorption test, or microhemagglutination assay for Treponema pallidum.13 As previously noted, selected articles were those published in English within the past 50 years, and disagreements, if any, were resolved by the third author.
Statistical analysis
Epilepsy prevalence among patients with syphilis: first, we summarized all data from the articles and made a table for further analysis. We then used R software (version 3.5.0) to analyze the prevalence of epileptic seizures among patients with syphilis. We used fixed- and random-effect models to determine the degree of heterogeneity among studies and fitted meta-regression models to assess the prevalence of epileptic seizures among patients with syphilis.14 Analyses was based on stratification into three subgroups to explore heterogeneity, according to the case collection time and the number of participant patients.
Case reports of epilepsy in patients with syphilis: we summarized patient data from all collected articles, and the details were extracted by the first author of our team and checked by the second. We classified the information based on the patients (age, sex and address), their health characteristics, the details of the epileptic seizure (seizure type, motor seizure or not and conscious state), additional tests (electroencephalogram findings and imaging tests) and prognosis. To assess the frequency of epileptic seizure symptoms in patients with syphilis, we also determined whether each patient had status epilepsy, and we classified the seizure type in accordance with the International League against Epilepsy (ILAE) 2017 classification.
Results
Literature search
Our search, using the keywords “syphilis and epilepsy,” identified 431 articles. Figure 1 is a flow diagram describing the selection of studies and their sources; 178, 5, 201, 2, 4 and 41 articles were selected from the PubMed, Cochrane Library, Embase, WHOLIS, SciELO and Web of Science databases, respectively. Clinicaltrails.gov had no relevant record. After the exclusion of duplicate and irrelevant studies, 49 articles were utilised for further analyses.5,7-10,15-58 Notably, we found only eight articles which discussed the prevalence of epileptic seizures in patients with syphilis; the remaining articles were case reports.8,10,24,35,40,54-56 The relationship between syphilis and epilepsy was assessed from two perspectives: a meta-analysis of the prevalence of epileptic seizures in patients with syphilis [Table 1] and a systematic review of clinical manifestations in these patients. In addition, five case reports described epileptic seizure as a symptom of the Jarisch-Herxheimer reaction.21,32,46,52 One article contained a case report and investigation of the prevalence of epilepsy in patients with syphilis; another article contained case reports of two patients with syphilis and epilepsy and a patient who exhibited the Jarisch-Herxheimer reaction; two case reports described two patients each, and another case report described three patients.10,16,36,37 Thus, we collected a total of 45 case reports describing patients with syphilis and epilepsy (excluding one case report that solely described electroencephalography findings57).

- The details of the source of the records and the choices of the matched literature
| Article | Syphilis with epileptic seizure | Syphilis | Frequency (%) |
|---|---|---|---|
| Tong et al. (2013) | 13 | 169 | 7.7 |
| Punia et al. (2013) | 22 | 178 | 12.4 |
| Sinha et al. (2008) | 30 | 119 | 25.2 |
| Mitsonis et al. (2008) | 3 | 81 | 3.7 |
| Timmermans et al. (2004) | 14 | 161 | 8.7 |
| Alani et al. (1982) | 1 | 21 | 4.8 |
| Sivaraju et al. (1976) | 68 | 282 | 24.1 |
| Hooshmand et al. (1972) | 58 | 241 | 24.1 |
Meta-analysis
A total of 1252 patients were included, among whom 209 had epileptic seizures. The frequency of epileptic seizures in patients with syphilis ranged from 4.8% to 25.2%. We used a random-effect model to analyze the prevalence of epileptic seizure in patients with syphilis, given the large degree of heterogeneity among studies; the proportion (95% confidence interval) ranged from 0.04 (0.01–0.10) to 0.24 (0.19–0.30), and the pooled estimate of proportion (95% confidence interval) and tau2 value were 0.1384 (0.0955–0.2005) and 0.2034, respectively [Figure 2]. For further analysis, the patients were divided into two groups according to case collection time (before and after 1985). One article summarized data from patients in both periods (1965–1984 and 1985–2005); thus, we divided patients in that study between the two groups in our analysis.35 Three articles that described studies of less than 150 patients were excluded because their I2 metrics showed a different degree of reduction.10,35,54 The A group included cases collected after 1985; in that group, the proportion (95% confidence interval) ranged from 0.03 (0.00–0.18) to 0.25 (0.18–0.34) and the proportion (95% confidence interval) in the random-effect model was 0.1132 (0.0644–0.1992). The evaluation indices of heterogeneity were tau2 = 0.3059, H = 2.49 (1.65–3.73) and I2 = 83.8% (63.5%–92.8%). The B group included cases collected before 1985; in that group, the proportion (95% confidence interval) ranged from 0.04 (0.00–0.13) to 0.24 (0.19–0.30), and the proportion (95% confidence interval) in the random-effects model was 0.1991 (0.1351–0.2933). The evaluation indices of heterogeneity were tau2 = 0.0756, H = 1.79 (1.05–3.04) and I2 = 68.7% (9.4%–89.2%). The C group comprised excluded articles with less than 150 patients; in that group, the proportion (95% confidence interval) ranged from 0.08 (0.04–0.13) to 0.24 (0.19–0.30) and the correlation values were 0.1439 (0.0938–0.2210), tau2 = 0.2016, H = 2.95 (2.03– 4.28) and I2 = 88.5% (75.8%–94.5%) [Figure 3].

- The forest plot of odds ratios for prevalence of epilepsy seizure in syphilis
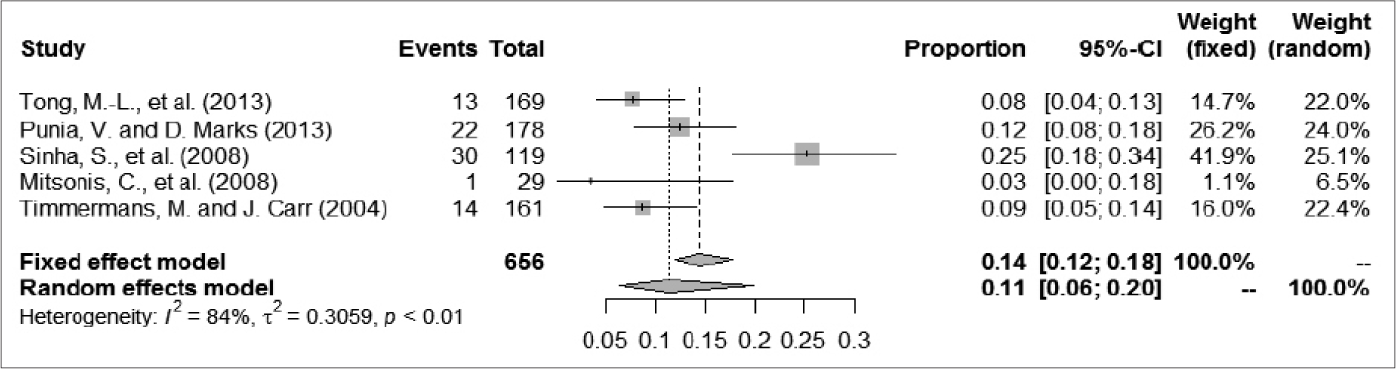
- The forest plots of odds ratios for prevalence of epilepsy seizure in syphilis: Collection time after 1985
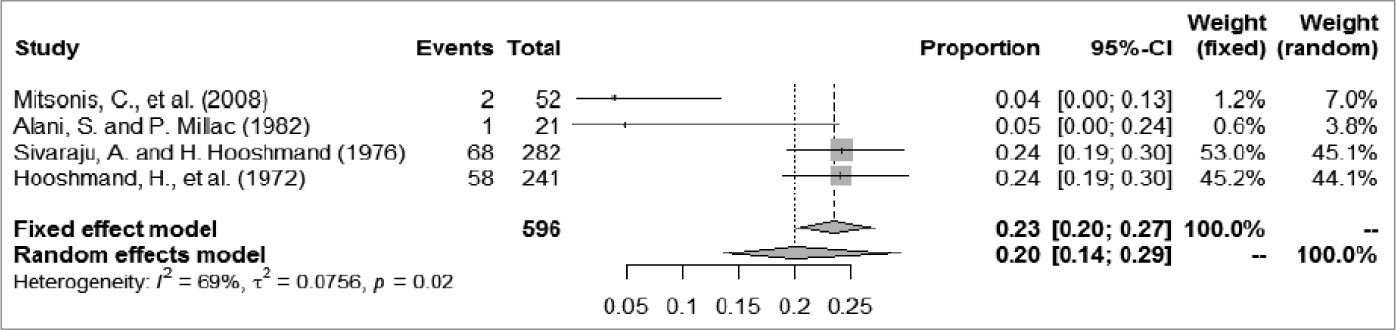
- The forest plots of odds ratios for prevalence of epilepsy seizure in syphilis: Collection time before 1985
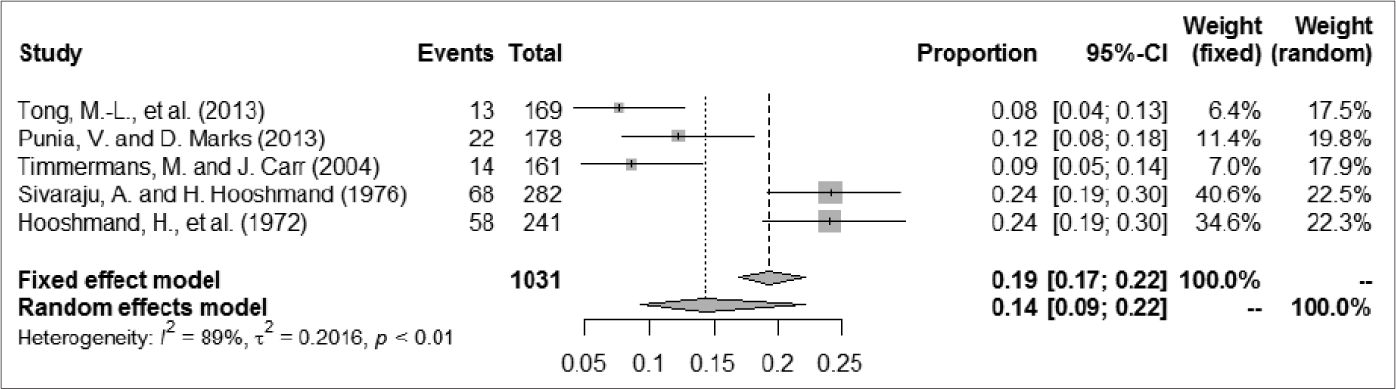
- The forest plots of odds ratios for prevalence of epilepsy seizure in syphilis: The number of patients more than 150
Case report and systematic review
We collected all patient information, including age, sex and region of residence. The patients were divided into seven groups based on their regional distribution. The patients were located in the following regions: North America (n = 12), Asia (n = 10), Europe (n = 8), Africa (n = 6). Latin America (n = 1), Australia (n = 1) and not clear (n = 2). Men were affected more often than women (31 vs. 9). The age range was 18–84 years, with a mean of 45.15 years; the mean ages in the regions examined ranged from 29.0 to 48.4 years, and the age distribution differed among regions. The follow-up period ranged from 20 days to 5 years; 37 (97.4%) patients were seizure-free, and one patient had a single recurrent tonic-clonic seizure during the 1st year of follow-up [Figure 4].48 Most patients recovered fully or developed only minor neurological sequelae, although four patients eventually showed severe gross functional deficits or died of the disease.7,18,31,34 Five patients exhibited the Jarisch-Herxheimer reaction after injection with penicillin (21–30 million units daily); only the oldest article described the use of a dose slightly exceeding that recommended in the guidelines.59 These patients had epileptic seizures >10 h after penicillin treatment; three of these patients were from North America, and the other two were from Asia (n = 1) and Oceania (n = 1). The affected patients were 40–71 years of age; one woman was 71 years of age and the other were men with age ranging from 40 to 57 years. The epilepsy types differed among the five patients, and the seizure type in one patient was a new one as defined by the author of another report.52,60 Electroencephalograms showed similar lateralized periodic discharges in three of the five patients.
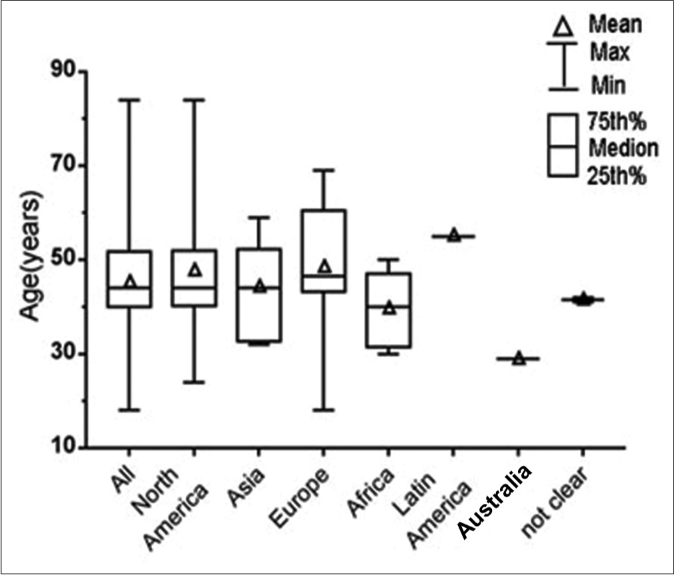
- The box and whisker plot of the ages of the patients in different regions
We analyzed epileptic seizure types among patients with syphilis from two perspectives to elucidate the common symptoms of this disease. Eighteen (45%) patients had status epilepticus and 22 (55%) did not; 32 (80%) patients had motor seizures and six (15%) had nonmotor seizures; the type of seizure (motor/nonmotor) was unclear for two (5%) patients because the articles did not clearly describe their symptoms; 20 (62.5%) patients in the motor seizure group had tonic-clonic seizures. Furthermore, 30 (75%) patients had impaired-awareness seizures, and five (12.5%) were aware; awareness status was not clear for five (12.5%) patients [Figure 5]. All patients had at least one positive reaction on syphilitic tests on blood and cerebrospinal fluid. The most common electroencephalography finding was epileptiform activity (21 of 25 patients); 11 (52.4%) of these 21 patients showed lateralized periodic discharges.
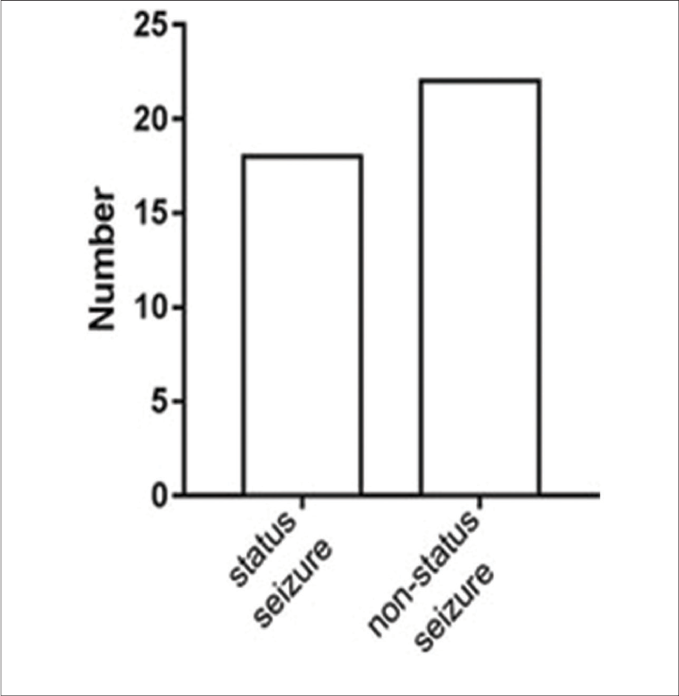
- The classification according to the International League against Epilepsy classification of the epilepsies position paper of the International League against Epilepsy commission: The patients are status seizure or nonstatus seizure
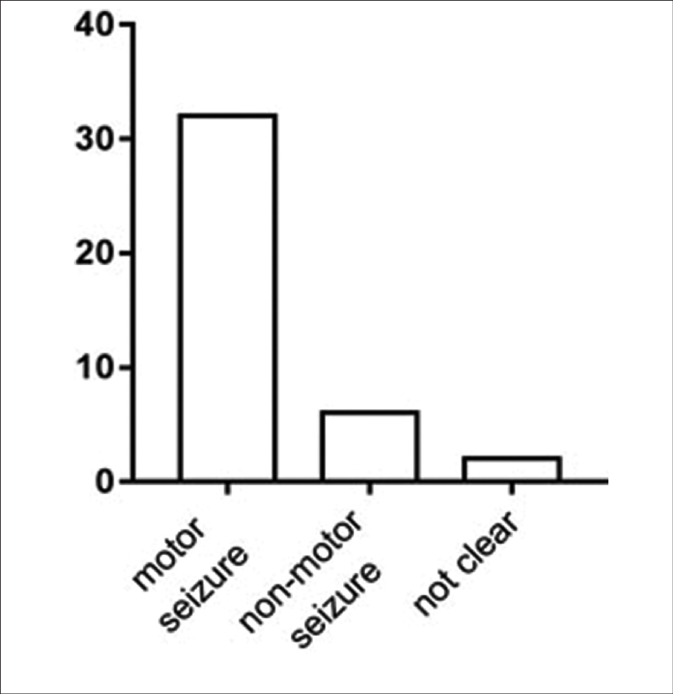
- The classification according to the International League against Epilepsy classification of the epilepsies position paper of the International League against Epilepsy commission: The patients have motor seizure or nonmotor seizure
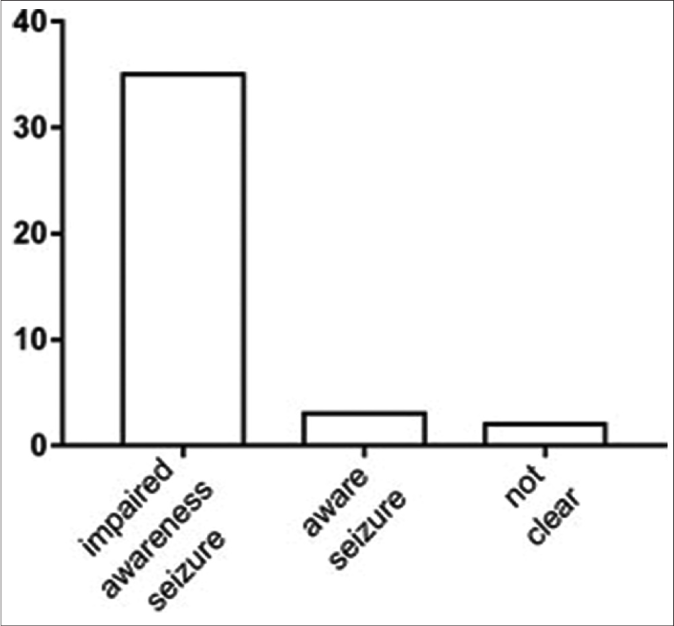
- The classification according to the International League against Epilepsy classification of the epilepsies position paper of the International League against Epilepsy commission: The patients are aware or impaired awareness
Discussion
Our study sought to clarify the prevalence of epileptic seizures caused by syphilis. The results suggested that the proportion of prevalence of epileptic seizures among patients with syphilis was 0.1384 in a random-effect model. In the subgroups, the prevalence in group A (articles published after 1985) was lower than that in group B. Over time, widespread antibiotic use and improved medical conditions may have contributed to this difference.35 After excluding three articles that described less than 150 patients, the prevalence in those articles was consistent with the overall findings of our analysis.
Syphilis involves multiple sites in the nervous system and therefore has protean manifestations, with the presentation ranging from a mild headache to coma and death. The clinical features have been considerably modified by the widespread use of antibiotics, and because of the inefficient treatment of syphilis, this disease often occurs in atypical forms or alters the clinical manifestation and obscures the diagnosis. Because of this, epilepsy is an extremely easily overlooked symptom in syphilis and is often misdiagnosed.8,10,35 Epileptic seizures occur in various phases, such as in syphilitic meningitis, meningovascular syphilis, general paralysis, and in the initial asymptomatic stage, but in the present time, the seizures are more often concentrated in the meningovascular stage. Because there is strong evidence of a positive relationship between treatment delay and seizure duration, the diagnosis and treatment are necessary and challenging.61 To identify the etiology as soon as possible, we identified the clinical features of epileptic seizures in patients with syphilis, according to the summarized relevant published case reports. Nearly half of the patients had status epilepsy, which is more than the proportion of such cases among all patients with epilepsy.61 The status seizure is an emergency requiring prompt treatment to reduce the risk of cerebral injury and subsequent morbidity and mortality; seizure cessation is less likely as time- to- therapy lengthens, and timely treatment for the majority of patients is still not achieved.61 It is important to identify the causes of epilepsy, especially for patients with syphilis, who may achieve a favorable prognosis with prompt treatment. According to our data, the largest proportion of patients with syphilis and epileptic seizure was in the middle-aged population and younger, consistent with a previous report in which nearly half of the patients were 35–50 years of age.40 These findings are consistent with the overall epidemiology of epilepsy.62 There was no significant difference in the mean and median age of patients in different regions. In addition, the proportion of male to female patients varied greatly among the included articles, with a mean of 3:1. This distribution may be explained by the increase in high-risk sexual intercourse (seamen, homosexuals, drug addicts) in males.35
We analyzed the seizure type from two perspectives, in accordance with the International League against Epilepsy classification position paper of the International League against Epilepsy Commission. In our systematic review, the most frequent seizure type among patients with syphilis was motor seizure with impaired awareness, which was often accompanied by tonic-clonic seizure; these results were similar to those reported by Sinha et al.10 On the other hand, in the additional tests, a large majority of patients had the typical electroencephalography signs of epileptiform activity. Interestingly, most patients with epileptiform activity showed the same type, namely lateralized periodic discharges, which has been associated with inflammatory and/or infectious diseases, stroke, brain tumors and metabolic derangements.10 The case report published by Markand and Daly, which was not included, also mentioned the electroencephalography finding of diffuse, slow background and lateralized periodic discharges.16,57 The electroencephalography can provide laboratory data supporting the diagnosis of epilepsy, but we cannot conclusively state that lateralized periodic discharges constitute the representative electroencephalography finding for patients with syphilis and epileptic seizure, and we emphasize the need for controlled clinical trials or larger confirmatory studies. There are no typical signs in neuroradiological findings in neurosyphilis, as has been shown in a previous South Indian study of 119 patients.8,10 During follow-up periods ranging from 20 days to five years, nearly all patients were seizure-free and few had neurological sequelae. Generally, the epileptic seizure does not affect recovery in most patients with syphilis, especially in those who receive effective treatment. Therefore, prompt diagnosis is necessary, combined with appropriate etiological treatment to prevent disease deterioration, thereby ensuring that all patients can recover.
Only five case reports described the Jarisch-Herxheimer reaction along with an epileptic seizure. This acute febrile inflammatory reaction often includes headache, myalgia, tachycardia, hyperventilation and worsening of lesions; few patients experience epileptic seizure as the initial or main symptom.63 In these five cases, seizures generally occurred >10 h after treatment with the recommended dose; symptomatic treatment was then provided; subsequently, the seizures stopped and the patients continued the appropriate anti-syphilis treatment. Clinicians should carefully monitor patients receiving anti-syphilis treatment to avoid the occurrence of the Jarisch-Herxheimer reaction.
Limitations
Although we searched all concerning articles published in the past nearly 50 years, we only included eight articles in the meta-analysis as research in this field has been conducted for a relatively short period. Furthermore, some patients with syphilis and epileptic seizure may not receive clear diagnoses. Our data was collected from case reports; thus, most patients may have had obvious symptoms and publication bias may exist, which may have affected the conclusions of the review. Thus, a significant knowledge gap remains with respect to the relationship between epilepsy and syphilis, and additional studies are needed to ascertain more clearly the prevalence and mechanisms underlying their association.
Conclusion
The prevalence of epileptic seizures, which was noted as one of the most overlooked and misdiagnosed manifestations in syphilis, is 13.8%. Mainly affected patients include middle-age men worldwide, who exhibit status epilepticus and motor seizure of the tonic-clonic type, with impaired awareness. In addition, the typical electroencephalography manifestation, lateralized periodic discharges, is an important sign in patients with syphilis and epileptic seizure. Although many articles describe both epilepsy and syphilis, most are case reports. In light of the limited data and a small number of articles, much additional research is necessary to elucidate the connection between syphilis and epilepsy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by the National Science Fund (#31770194, to Shuping Hou), China.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- The association between dementia and epilepsy: A systematic review and meta-analysis. Epilepsia. 2017;58:962-72.
- [CrossRef] [PubMed] [Google Scholar]
- Increased risk of hospital admission for mood disorders following admission for epilepsy. Neurology. 2018;91:e800-10.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: A 30-year longitudinal cohort study. JAMA Neurol. 2018;75:279-86.
- [CrossRef] [PubMed] [Google Scholar]
- Use of the ketogenic diet to treat intractable epilepsy in mitochondrial disorders. J Clin Med. 2017;6:E56.
- [CrossRef] [PubMed] [Google Scholar]
- Nonconvulsive status epilepticus associated with periodic lateralized epileptiform discharges in a patient with syphilis. Interdiscipl Neurosurg Adv Techn Case Manage. 2016;5:35-7.
- [CrossRef] [Google Scholar]
- Epilepsy in children: From diagnosis to treatment with focus on emergency. J Clin Med. 2019;8:E39.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis in an 18-year-old female patient: Case report. J Neurol. 2010;257:S211.
- [Google Scholar]
- Laboratory findings in neurosyphilis patients with epileptic seizures alone as the initial presenting symptom. Diagn Microbiol Infect Dis. 2013;75:377-80.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis masquerading as hemiparesis and Jacksonian epilepsy in an HIV positive patient: A case report. Afr Health Sci. 2010;10:211-4.
- [Google Scholar]
- Symptomatic seizures in neurosyphilis: An experience from a university hospital in South India. Seizure. 2008;17:711-6.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital syphilis and other STIs rise in the USA. Lancet Infect Dis. 2018;18:1186-7.
- [CrossRef] [Google Scholar]
- Syphilis trends in the central savannah river area (CSRA) of Georgia and South Carolina, USA. J Clin Med. 2018;7:E190.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of 2 reverse syphilis testing algorithms in diagnosis of syphilis: A large-cohort prospective study. Clin Infect Dis. 2018;67:947-53.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: Time is brain? Neurology. 2017;89:506-16.
- [CrossRef] [PubMed] [Google Scholar]
- Early neurosyphilis presenting with facial palsy and an oral ulcer in a patient who is human immunodeficiency virus positive: A case report. J Med Case Rep. 2017;11:134.
- [CrossRef] [PubMed] [Google Scholar]
- Periodic lateralized epileptiform discharges (PLEDs) in patients with neurosyphilis and HIV infection. Clin EEG Neurosci. 2016;47:247-50.
- [CrossRef] [PubMed] [Google Scholar]
- Two cases of oligosymptomatic neurosyphilis in immunocompetent patients: Atypical neurosyphilis presentation. Int J STD AIDS. 2016;27:155-6.
- [CrossRef] [PubMed] [Google Scholar]
- 'The great imitator': Neurosyphilis and new-onset refractory status epilepticus (NORSE) syndrome. Epilepsy Behav Case Rep. 2015;3:33-5.
- [CrossRef] [PubMed] [Google Scholar]
- Fulminant neurosyphilis and concurrent acute HIV infection presenting with new onset seizures. Eur J Neurol. 2014;21:432.
- [Google Scholar]
- Reversible dementia with myoclonus due to concurrent HSV-2 and syphilis central nervous system infection in an immunocompetent man. Neurology. 2014;82:P5.230.
- [Google Scholar]
- Stroke after initiating IV penicillin for neurosyphilis: A possible Jarisch-Herxheimer reaction. Case Rep Neurol Med. 2014;2014:548179.
- [CrossRef] [PubMed] [Google Scholar]
- A patient with progressive cognitive decline and periodic abnormal waves in EEG: PLEDs of neurosyphilis or PSDs of Creutzfeldt-Jakob disease? Clin EEG Neurosci. 2014;45:218-21.
- [CrossRef] [PubMed] [Google Scholar]
- The 'great imitator': An unexpected case of neurosyphilis. J Neurol Neurosurg Psychiatry. 2014;85:A31.
- [CrossRef] [Google Scholar]
- Clinical and EEG characteristics of seizure disorders in neurosyphilis. Neurology. 2013;80:P07-174.
- [CrossRef] [Google Scholar]
- A case of neuropsyphilis presenting as multiple ring enhancing lesions mimicking cerebral abscesses. Neurology. 2013;80:P04.015.
- [Google Scholar]
- Syphilitic limbic encephalitis revealed by status epilepticus. BMJ Case Rep. 2013;2013:bcr2012008073.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis presenting with psychotic symptoms and status epilepticus. Neurol Sci. 2012;33:99-102.
- [CrossRef] [PubMed] [Google Scholar]
- An unusual cause for hyponatremia with seizures. BMJ Case Rep. 2012;2012:bcr0920114784.
- [CrossRef] [PubMed] [Google Scholar]
- Late-stage neurosyphilis presenting with severe neuropsychiatric deficits: Diagnosis, therapy, and course of three patients. J Neurol. 2012;259:720-8.
- [CrossRef] [PubMed] [Google Scholar]
- A neurosyphilis case presenting with cognitive dysfunction, epileptic seizures, high signal intensity and significant atrophy in left amygdala/ hipocampal region. Klinik Psikofarmakoloji Bulteni. 2012;22:S156.
- [Google Scholar]
- Deterioration of MRI findings related to Jarisch-Herxheimer reaction in a patient with neurosyphilis. J Neurol. 2011;258:699-701.
- [CrossRef] [PubMed] [Google Scholar]
- Status epilepticus revealing syphilitic meningoencephalitis. Acta Neurol Belg. 2010;110:263-7.
- [Google Scholar]
- Incidence and clinical presentation of neurosyphilis: A retrospective study of 81 cases. Int J Neurosci. 2008;118:1251-7.
- [CrossRef] [PubMed] [Google Scholar]
- Periodic lateralized epileptiform discharges in neurosyphilis. Epilepsia. 2007;48:390-3.
- [CrossRef] [PubMed] [Google Scholar]
- Status epilepticus as an initial manifestation of neurosyphilis: A case report. Kaohsiung J Med Sci. 2006;22:404-9.
- [CrossRef] [Google Scholar]
- Neurosyphilis presenting with status epilepticus. Neurologist. 2006;12:314-7.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004;75:1727-30.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis with complex partial status epilepticus and mesiotemporal MRI abnormalities mimicking herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 2004;75:833.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis and status epilepticus: Case report and literature review. Epilepsy Res. 2004;59:67-70.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated episodes of status epilepticus as the manifestation of neurosyphilis: A case report. Epilepsia. 2003;44:623.
- [CrossRef] [PubMed] [Google Scholar]
- Status epilepticus presenting in a patient with neurosyphilis and a previously asymptomatic arachnoid cyst. Epilepsia. 2002;43:775-6.
- [CrossRef] [PubMed] [Google Scholar]
- Syphilitic meningitis in HIV-patients with meningeal syndrome: Report of two cases and review. Braz J Infect Dis. 2001;5:280-7.
- [CrossRef] [PubMed] [Google Scholar]
- Nonconvulsive status epilepticus resulting from Jarisch-Herxheimer reaction in a patient with neurosyphilis. Clin Electroencephalogr. 2000;31:138-40.
- [CrossRef] [PubMed] [Google Scholar]
- Intractable epilepsy as the initial manifestation of neurosyphilis. Epilepsia. 1999;40:1309-11.
- [CrossRef] [PubMed] [Google Scholar]
- De novo status epilepticus as the presenting sign of neurosyphilis. Epilepsia. 1998;39:1367-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral syphilitic gumma in an HIV-negative patient presenting as prolonged focal motor status epilepticus. N Engl J Med. 1996;335:1159-60.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis presenting as complex partial status epilepticus. Eur Neurol. 1996;36:111-2.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis presenting as refractory status epilepticus. Am J Emerg Med. 1995;13:685-6.
- [CrossRef] [Google Scholar]
- Jarisch-Herxheimer reaction in a patient with neurosyphilis: Non-convulsive status epilepticus? J Neurol Neurosurg Psychiatry. 1995;58:521.
- [CrossRef] [PubMed] [Google Scholar]
- Periodic EEG pattern in meningovascular syphilis. J Neurol Neurosurg Psychiatry. 1984;47:1360-1.
- [CrossRef] [PubMed] [Google Scholar]
- Neurosyphilis in the Leicester area. Postgrad Med J. 1982;58:685-7.
- [CrossRef] [PubMed] [Google Scholar]
- Seizure disorders associated with neurosyphilis. Case Rep Neurol Med. 1976;37:133-6.
- [Google Scholar]
- Pseudoperiodic lateralized paroxysmal discharges in electroencephalogram. Neurology. 1971;21:975-81.
- [CrossRef] [PubMed] [Google Scholar]
- 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2014;28:1581-93.
- [CrossRef] [PubMed] [Google Scholar]
- Jarisch-Herxheimer reaction in a patient with neurosyphilis. J Neurol Neurosurg Psychiatry. 1994;57:865-7.
- [CrossRef] [PubMed] [Google Scholar]
- Timing is everything: Where status epilepticus treatment fails. Ann Neurol. 2017;82:155-65.
- [CrossRef] [PubMed] [Google Scholar]
- Active epilepsy and seizure control in adults-United States 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67:437-42.
- [CrossRef] [PubMed] [Google Scholar]
- The Jarisch-Herxheimer reaction in syphilis: Could molecular typing help to understand it better? J Eur Acad Dermatol Venereol. 2018;32:1791-5.
- [CrossRef] [PubMed] [Google Scholar]






