Translate this page into:
Non-infective granulomatous dermatitis following Mycobacterium w injections
Corresponding author: Dr. Chirag Desai, Dadar, B21, Krishnalaya Society, 6th Floor, N.S.Mankikar Marg, Chunabhatti-west, Mumbai, Maharashtra, India. chirag@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Desai C, Gala S. Non-infective granulomatous dermatitis following Mycobacterium w injections. Indian J Dermatol Venereol Leprol 2022;88:829-31.
Sir,
The recent COVID-19 infection caused a worldwide pandemic, the effects of which have not still subsided even after the development of vaccines, due to various mutations in the virus. Coronaviruses are enveloped non-segmental positive-sense RNA viruses, belonging to the Coronaviridae family and Nidovirales order. COVID-19 infection causes mild to severe respiratory illness. A severe acute respiratory syndrome due to this infection results in critical illness with intensive care unit admission and mortality.1 Several measures were tried to revive such patients, one of which was the administration of Mycobacterium w injections. We report a case presenting as an exaggerated local side effect of this vaccine.
A 45-year-old woman presented with multiple asymptomatic lesions on the abdomen and both upper arms for six weeks. She had taken several antibiotics without any benefit. There was a history of intensive care unit admission two months back for severe COVID-19 infection. On enquiry, she revealed that she was given certain injections at the site of lesions during her hospital stay. As per her discharge summary, it was found that these were Mycobacterium w injections. On cutaneous examination, there were multiple non-tender, erythematous ulcerated plaques, with some oozing and crusting on the surface. They were distributed mainly on the abdomen and both upper arms [Figure 1]. A provisional diagnosis of atypical mycobacterial infection, deep fungal infection and ecthyma were considered. Skin biopsy revealed granulomatous dermatitis with tuberculoid granulomas in the upper reticular dermis and suppurative granulomas in the lower reticular dermis [Figures 2 to 4] Specials stains like periodic acid-Schiff, Ziehl-Neelsen stain and Fite stain did not demonstrate any organisms. She denied a second biopsy for culture study. A diagnosis of non-infective granulomatous dermatitis was given based on the histopathology features and side effects mentioned in the literature.2 She was started on 20 mg prednisolone and the lesions subsided within one month leaving behind post-inflammatory scarring [Figure 5]. She is on regular follow up with no recurrence. Mycobacterium w or Mycobacterium indicus pranii injections are immunomodulatory injections which contain heat-killed organism and it is given intradermally at three different sites daily for three days in 0.1 mL dose to severely ill patients with gram-negative sepsis admitted in the intensive care unit. Other indications include lepromin-negative leprosy and non-small cell lung cancer patients along with paclitaxel and cisplatin regimens.
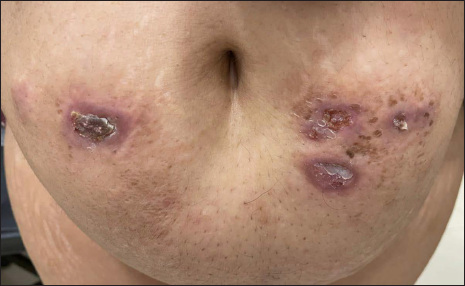
- Multiple erythematous ulcerated plaques, few showing crusts on surface at injection sites
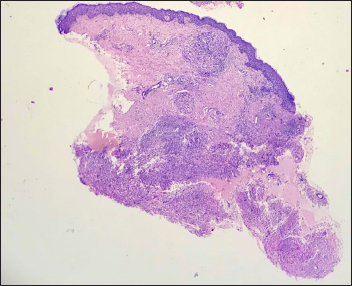
- Tuberculoid and suppurative granulomatous dermatitis (H&E ×40)
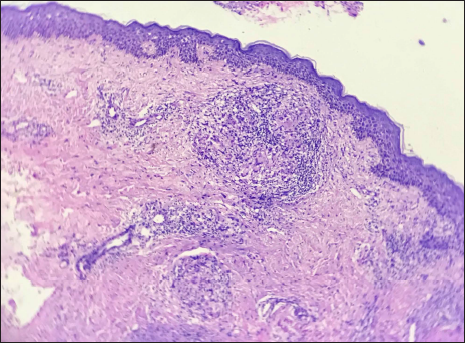
- Tuberculoid granuloma in upper dermis (H&E ×100)
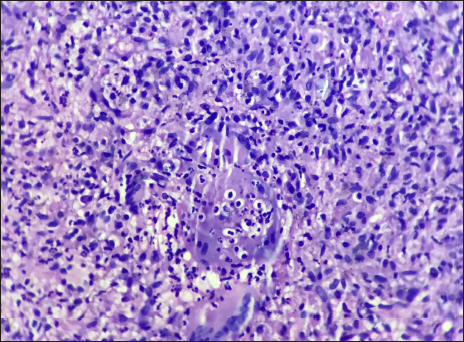
- Suppurative granuloma in lower dermis (H&E ×400)
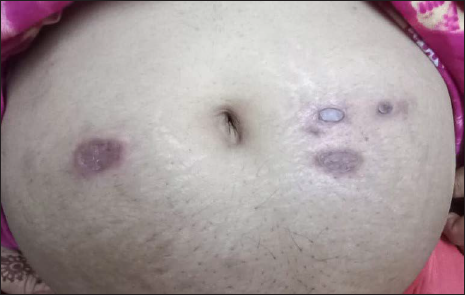
- Lesions healing with treatment
At the advent of the second wave, Mycobacterium w injections were given to severely ill COVID-19 infected patients admitted to the intensive care unit. This was in accordance with the guidelines laid down by the Global Research Collaboration for Infectious Disease Preparedness and the World Health Organization, which suggested that adjuvant therapy is one of the methods to save patients infected with severe COVID-19 infection.2 Mechanism of action of Mycobacterium w vaccine as studied in leprosy was via toll-like receptors, thus enhancing the host T cell responses. It is known to be safe and well-tolerated in gram-negative sepsis and the recent report also suggests the same for severe COVID-19 infection.3
Ingale et al. in their study on the use of Mycobacterium w vaccine for severely ill COVID-19 patients, reported local site reaction in 85.4% of patients, out of which the majority (54%) were mild and a few (14%) were severe. It comprised erythema and induration, followed by pustule formation and ulceration which healed by scarring. Mild reactions did not require any major treatment, however severe reactions required oral anti-inflammatory agents and topical steroids.3
Injection site granulomatous reaction to immunotherapeutic agents like interferons4 and vaccines5-7 is a known phenomenon. The usual injection site side effect of Mycobacterium w vaccine is the formation of asymptomatic papule/plaque or tender nodule which later ulcerates and heals with atrophic scarring. Khullar et al. have reported a generalised granulomatous dermatitis in two of their lepromatous leprosy patients with Mycobacterium w vaccine. In their cases, the lesions were seen distant to the injection site, with the persistence of ulceration. They hypothesise that the generalised lesions could be a manifestation of haematogenous spread or strong delayed-type hypersensitivity to Mycobacterium w antigens6. Sharma et al. in their study evaluating the safety and efficacy of usage of Mycobacterium w vaccine as adjunctive therapy in category 2 pulmonary tuberculosis reported injection site reaction in 82.4% of the patients. About 68% of the patients experienced a mild intensity of the reaction whereas 12.9% had moderate to severe reaction at the local site. These reactions healed spontaneously, without active treatment.7
In our case, typical history, characteristic clinical features, no organisms found on special staining and response to anti-inflammatory agents clinched the diagnosis. There are relatively fewer reports in the literature demonstrating the histopathology features of this side effect when Mycobacterium w vaccine was used as adjunctive therapy in COVID-19 patients. Our purpose of reporting this case was to create awareness among the dermatologists and pathologists about the local side effects of these injections and to avoid unnecessary treatment with multiple antibiotics.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium W. An unusual side effect. Indian J Tuberc. 2021;69:250-2.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Mycobacterium w for the treatment of COVID-19: An observational study. J Assoc Physicians India. 2021;69:19-22.
- [PubMed] [Google Scholar]
- Cutaneous granulomas associated with interferon therapy. Am J Dermatopathol. 2016;38:892-9.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional injection of Mycobacterium w vaccine vs imiquimod, 5%, cream in patients with anogenital warts: A randomized clinical trial. JAMA Dermatol. 2014;150:1072-8.
- [CrossRef] [PubMed] [Google Scholar]
- Generalized granulomatous dermatitis following Mycobacterium w (Mw) immunotherapy in lepromatous leprosy. Dermatol Ther. 2017;30
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and Safety of Mycobacterium indicus pranii as an adjunct therapy in Category II pulmonary tuberculosis in a randomized trial. Sci Rep. 2017;7:3354.
- [CrossRef] [PubMed] [Google Scholar]





