Translate this page into:
Low dose oral glucocorticoid therapy in lichen planus: A retrospective cohort study
Corresponding author: Dr. Kamilla Koszorú, Department of Dermatology, Venereology, and Dermatooncology, Semmelweis University, Budapest, Hungary. koszoru.kamilla@med.semmelweis-univ.hu
-
Received: ,
Accepted: ,
How to cite this article: Koszorú K, Kovács A, Lőrincz K, Medvecz M, Sárdy M. Low dose oral glucocorticoid therapy in lichen planus: A retrospective cohort study. Indian J Dermatol Venereol Leprol 2023;89:568–71.
Abstract
Background
There are various topical and systemic treatment options for the management of lichen planus. However, it is often difficult to achieve long-term disease control and many of the common therapies may be associated with unwanted side effects.
Aims
To evaluate the effectiveness of 8 mg oral methylprednisolone administered daily in lichen planus by the analysis of medical records.
Methods
In this retrospective cohort study, we compared the rates of improvement between two groups of patients. The first group received 8 mg oral methylprednisolone daily for at least one month. In the second group, patients with similar parameters to the first group (age, sex, disease manifestation) but without systemic glucocorticoid therapy were included. Fisher’s exact test was used to compare the rates of remission in the two groups.
Results
In the daily oral methylprednisolone (n = 24) and no systemic corticosteroids (n = 16) groups, 23 (95.8%) and 6 (37.5%) patients achieved partial or complete remission, respectively. The frequency of improvement was significantly higher in patients who received oral methylprednisolone (P < 0.0001).
Limitations
Limitations of this study include its retrospective design and the relatively small sample size.
Conclusion
Low dose oral glucocorticoid therapy may be an effective option for the systemic treatment of lichen planus. Based on our results and previous studies, instead of higher doses, longer therapy duration with low doses should be considered.
Keywords
Lichen planus
oral lichen planus
systemic treatment
glucocorticoids
corticosteroids
Plain Language Summary
Lichen planus is a disease that causes inflammation of the skin, nails and/or mucous membranes. There are several treatment options, however, most of these do not provide long-term disease control, some of the medications may cause severe side effects, and the application of creams or ointments every day is inconvenient and time-consuming. In this study, we investigated a new treatment approach by comparing two groups of patients. We found that significantly more patients achieved improvement in the group where low dose oral glucocorticoids were administered once daily. In the other group, participants received treatments other than glucocorticoids. This is relevant because conventionally, in lichen planus, glucocorticoids have been used at higher doses which can potentially result in serious adverse effects. Nevertheless, if administered in low doses, glucocorticoids are fairly safe, thus therapy can be maintained until disease control is achieved. Overall, the results of this study support the utility of low dose oral glucocorticoids in the treatment of lichen planus.
Introduction
Lichen planus is a chronic mucocutaneous inflammatory disease. The pathomechanism is not completely understood; however, an autoimmune process mediated by autoreactive T-lymphocytes has been proposed.1,2 Itch in cutaneous lichen planus and pain in erosive oral lichen planus substantially restrict patients’ quality of life, but achieving remission remains a challenge. Topical glucocorticoids are the first-line treatment. Narrow-band ultraviolet B(UVB) is also frequently utilised; however, external therapy is usually not sufficient to establish long-term disease control. Oral retinoids are one of the first-line systemic therapies, but potential adverse effects restrict their applicability.3 Further options are oral glucocorticoids which are generally administered in high doses (0.5-1 mg/kg/day prednisone equivalent), with a high risk of causing iatrogenic Cushing’s syndrome.4 Alternatively, other immunosuppressants (e.g., azathioprine, methotrexate) may be considered, but possible adverse effects limit their use, too.4
The senior author of this article observed that a high percentage of lichen planus patients receiving a mid-term, low dose, slowly tapered regime of oral glucocorticoid therapy often recover completely. Our hypothesis was that 8 mg of oral methylprednisolone daily, administered for at least one month, can effectively improve the signs and symptoms of lichen planus. This is equivalent to 10 mg prednisone, which is regarded as a low, anti-inflammatory dose and corresponds to the Cushing’s threshold, i.e., the dose at or under which the risk for iatrogenic Cushing’s syndrome is minimal.5 Our goal was to assess the effectiveness of this treatment strategy by comparing the frequency of partial or complete recovery in patients treated with 8 mg methylprednisolone with patients who did not receive systemic glucocorticoids.
Methods
This was a retrospective cohort study conducted on adult patients with lichen planus. Ethical clearance was granted by the Regional and Institutional Committee of Science and Research in Hungary (March 1, 2021, reference No.: 34/2021). We analysed medical records from 2016 to 2020: patients with cutaneous lichen planus and/or oral lichen planus were selected. In oral lichen planus, the diagnosis had to be confirmed by histology; in cutaneous lichen planus, histology was not required if the clinical presentation was typical. The exposed group comprised of patients who received oral methylprednisolone treatment for at least one month with a starting dose of 8 mg/day (low dose oral daily methylprednisolone group, oral daily methylprednisolone). Patients treated with any other systemic glucocorticoid regimen were excluded. The exposed group included patients treated with low dose oral methylprednisolone monotherapy as well as those who received low dose methylprednisolone along with other concomitant treatment. The unexposed group consisted of patients who did not receive low dose oral methylprednisolone but matched the exposed group in terms of age, sex, diagnosis as well as nature and duration of other concomitant treatment received.
The primary outcome was clinical improvement. Patients were deemed to have achieved partial or complete remission if one of the following terms was used in the medical records of the last visit: “improvement,” “only residual lesions,” “no skin lesions,” “no mucosal lesions” or “lichen in complete remission.” The latter four terms defined complete remission. An assessment of ‘no improvement’ was made if the medical records at the last visit stated “no improvement of lesions,” “progression of lesions” or “new lesions appeared”. The latter two terms determined progression. As some patients were lost to follow-up after treatment, those who achieved remission were contacted over the phone, and the long-term outcome was analysed based on verbal information. Exclusion criteria were uncertain diagnosis, lost to follow-up within one month, missing data about the therapeutic effect, and poor adherence to treatment.
Outcome data in the oral daily methylprednisolone and no systemic corticosteroid groups were compared by Fisher’s exact test. A P value of <0.05 was considered statistically significant. Relative risk was calculated to compare the chances of partial or complete recovery in the two groups.
Results
The demographic and clinical characteristics of subjects are summarised in [Table 1]. The oral daily methylprednisolone group (n=24) included patients with cutaneous (n = 10), mucosal (n = 7), mucocutaneous (n = 6) involvement and hypertrophic lichen planus (n = 1). Duration of treatment ranged from 1 to 16 months (mean: 4.25, median: 3.75; [Figure 1], and the dosage was tapered gradually and slowly (note that the rate of tapering was variable and it could not be standardised in this retrospective study). Sixteen patients received additional treatment besides methylprednisolone including topical glucocorticoids (n = 12), narrow-band UVB (n = 2), acitretin (n = 1) and methotrexate (n = 1).
| NSCS | ODMP | |
|---|---|---|
| Demographic data | ||
| No. of subjects (males/females) | 16 (0/16) | 24 (1/23) |
| Mean age (years) ± SD | 63.3 ± 7.6 | 63.0 ± 10.0 |
| Diagnosis | ||
| Cutaneous LP | 7 | 10 |
| Oral LP | 4 | 7 |
| Cutaneous and oral LP | 4 | 6 |
| Hypertrophic LP | 1 | 1 |
| Treatment (no. of subjects) | ||
| Methylprednisolone 8 mg | − | 24 |
| Topical glucocorticoid | 12 | 12 |
| NB-UVB | 2 | 2 |
| Acitretin | 1 | 1 |
| Methotrexate | 1 | 1 |
| Methylprednisolone therapy duration (months)* | ||
| Mean ± SD | − | 4.25 ± 3.58 |
| Median | − | 3.75 |
| Minimum | − | 1 |
| Maximum | − | 16 |
| Response to treatment | ||
| Partial remission | 3 | 14 |
| Complete remission | 3 | 9 |
| No improvement | 9 | 1 |
| Progression | 1 | 0 |
| Relapse after last follow-up visit in those who achieved partial or complete remission | 4 (n = 6) | 17 (n = 23) |
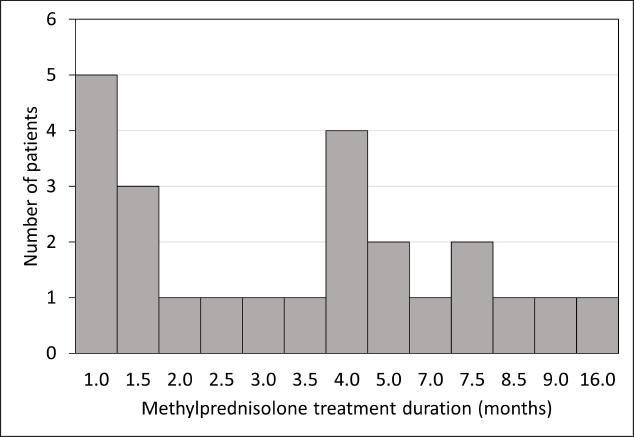
- Distribution of treatment duration in the oral daily methylprednisolone (ODMP) group (n = 24)
In the oral daily methylprednisolone group, 14 (58.3%) patients achieved partial remission, 9 (37.5%) achieved complete remission, 1 (4.2%) showed no improvement, and none had progression [Table 1]. The subject with no improvement had oral lichen planus and received methylprednisolone for five months supplemented with topical glucocorticoids for two months. In the no systemic corticosteroids group, 3 patients (18.8%) had partial remission, 3 (18.8%) had complete remission, 9 (56.2%) did not improve, and 1 (6.2%) had progression. Patients with improvement in the no systemic corticosteroids group received topical glucocorticoids (n = 3), narrow-band UVB (n = 1), acitretin (n = 1), or methotrexate (n = 1). Those who showed no improvement used topical glucocorticoids (n = 9) and the patient with progression received narrow-band UVB.
Among those who achieved improvement, 17 (73.9%) had a relapse after the end of treatment in the oral daily methylprednisolone group and 4 (66.7%) in the no systemic corticosteroids group. No severe adverse events occurred. In the oral daily methylprednisolone group, four patients reported gastric complaints, most of which could be controlled by antacids, slightly elevated blood pressure was detected in two patients, and headache or mild oedema were reported by one patient each.
Subjects receiving oral methylprednisolone had a higher chance of partial or complete recovery than those who received no systemic glucocorticoids (relative risk = 2.57, 95% confidence interval: 1.35-4.84). Improvement in general (including both partial and complete remission) occurred significantly more frequently in the oral daily methylprednisolone group P < 0.0001, [Figure 2].
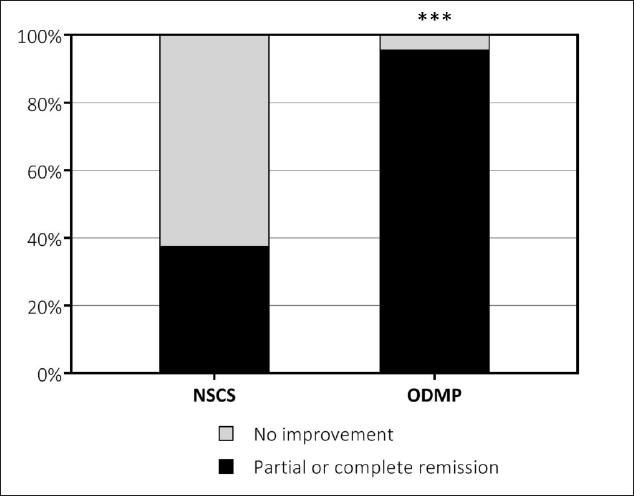
- Ratio of remission in the no systemic corticosteroids (NSCS, n = 16) and oral daily methylprednisolone (ODMP, n = 24) groups. ***Significant difference, P < 0.0001
Discussion
When prescribing systemic glucocorticoids, it is recommended to use the minimal effective dose or to limit treatment duration to minimise the risk of adverse effects.6 This study presents a safe and potentially effective treatment approach for lichen planus.
According to a recent systematic review, eight studies had been conducted investigating the effectiveness of oral glucocorticoids in cutaneous lichen planus.7 The treatment regimens were highly variable. High or moderate doses (0.3-1 mg/kg/day prednisone or prednisolone) were mostly used over short periods and showed either no benefit compared to placebo and narrow-band UVB or caused more adverse effects than methotrexate and enoxaparin.8-11 Another approach was treatment with systemic glucocorticoids having retarded effect. In one study, betamethasone 2×5 mg/week (i.e., a total of 62 mg prednisolone equivalent weekly) was found to be as effective as methotrexate 10 mg/week, with slightly more adverse effects.12 This approach is similar to ours (a continuous, low dose glucocorticoid regimen) with the difference that methylprednisolone is given daily, not weekly because of its pharmacokinetics.
In oral lichen planus, the application of topical products on the oral mucosa is time-consuming and inconvenient, often resulting in poor treatment adherence. Therefore, more convenient, yet safe treatment options are necessary. Only a few studies have investigated systemic glucocorticoids in oral lichen planus.13 An open trial compared oral prednisone (50 mg/day) with topical clobetasol ointment: no significant difference was found in therapeutic effect, but a greater risk for systemic side effects in the prednisone group was reported.14 Similarly, oral betamethasone 2 × 5 mg/week was found to be an equally effective alternative to topical triamcinolone paste in a randomised controlled trial.15
When reviewing the literature, we found a lack of consensus on the treatment strategy with systemic glucocorticoids. The disadvantage of high doses is that they cannot be administered long enough to achieve proper disease control without causing adverse effects. Based on our results and previous studies, low dose treatment may be a potentially effective, yet safe alternative, while high doses do not provide significantly greater benefit. We demonstrated that doses corresponding to the Cushing’s threshold may be sufficient to reach disease control without increasing the risk of adverse effects. It is also more convenient compared to the application of topical products thus, better treatment adherence can be expected. Nonetheless, monitoring for adverse effects and calcium plus vitamin D supplementation is recommended if prolonged treatment occurs, even with low doses.6
This study has some limitations. Given the retrospective design, two major sources of potential bias were the non-randomised and non-blinded designs. Patients were not treated by a standard protocol; the rate of tapering was variable, and follow-up was performed in a non-blinded way without an objective score system. Further limitations include the small sample size and the fact that data about disease control after treatment was partly self-reported. However, the strength of the study is that real-life data were collected without instructing physicians on how to manage and document patients. As the physicians could not know about this retrospective analysis, they were not biased in the evaluation of improvement.
Conclusion
The high remission rate achieved with 8 mg/day methylprednisolone suggests that it is an advantageous treatment strategy even despite the high relapse rate observed because prolonged administration is possible without causing severe adverse effects and the relatively high relapse rate may be avoided by longer treatment duration and slower tapering. Based on our results and previous studies, longer therapy duration with low doses of glucocorticoids should be considered instead of higher doses. There is a strong need for well-designed studies investigating this approach with regard to efficacy, side effects, and long-term disease control.
Declaration of patient consent
Patients’ consent was not required as patients’ identities are not disclosed or compromised.
Financial support and sponsorship
Kamilla Koszorú’s work was supported by Semmelweis University 250+ Excellence PhD Scholarship: EFOP-3.6.3-VEKOP-16-2017-00009.
Conflict of interest
There are no conflicts of interest.
References
- Cloned auto-Ia-reactive T cells elicit lichen planus-like lesion in the skin of syngeneic mice. J Immunol. 1986;137:2485-95.
- [PubMed] [Google Scholar]
- Lichen planus - a clinical guide. J Dtsch Dermatol Ges. 2021;19:864-82.
- [CrossRef] [PubMed] [Google Scholar]
- Lichen ruber planus: Besser verstehen, besser behandeln! [Lichen ruber planus: Better understanding, better treatment!] Hautarzt. 2018;69:100-08.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-32.
- [CrossRef] [PubMed] [Google Scholar]
- Systemische Therapie bei Hauterkrankungen In: Braun-Falco’s DermatologieVenerologie und Allergologie (7th ed.). Berlin: Springer; 2018. p. :2070-74. [Braun-Falco’s Dermatology Venereology and Allergology]
- [Google Scholar]
- Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457-65.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of cutaneous lichen planus (part 2): A review of systemic therapies. J Dermatolog Treat. 2019;30:633-47.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of lichen planus with a short course of oral prednisolone. Br J Dermatol. 1990;123:550-51.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial. J Res Med Sci. 2011;16:1578-82.
- [PubMed] [PubMed Central] [Google Scholar]
- A prospective study comparing therapeutic efficacy and safety of oral methotrexate and oral prednisone in the treatment of generalized cutaneous lichen planus. IOSR J Dent Med Sci. 2017;16:143-7.
- [CrossRef] [Google Scholar]
- Comparison of therapeutic effect of low-dose low-molecular-weight heparin (enoxaparin) vs. oral prednisone in treatment of patients with lichen planus; A clinical trial. Adv Biomed Res. 2013;2:76.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse outcome of methotrexate and mini pulse betamethasone in the treatment of lichen planus. Bangladesh Med Res Counc Bull. 2013;39:22-27.
- [CrossRef] [PubMed] [Google Scholar]
- Interventions for treating oral lichen planus: Corticosteroid therapies. Cochrane Database Syst Rev. 2020;2:CD001168.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic and topical corticosteroid treatment of oral lichen planus: A comparative study with long-term follow-up. J Oral Pathol Med. 2003;32:323-29.
- [CrossRef] [PubMed] [Google Scholar]
- Betamethasone oral mini-pulse therapy compared with topical triamcinolone acetonide (0.1%) paste in oral lichen planus: A randomized comparative study. J Am Acad Dermatol. 2008;58:596-602.
- [CrossRef] [PubMed] [Google Scholar]






