Translate this page into:
Association between asthma, rhinitis and atopic dermatitis with leprosy: A case-control study
Corresponding author: Prof. Marcio Bezerra-Santos, Medicine, Immunology and Molecular Biology Laboratory, University Hospital, Universidade Federal de Sergipe, SE, Brazil. marciobezerra.ufs@outlook.com
-
Received: ,
Accepted: ,
How to cite this article: Tenório MDL, Araujo JMS, de Melo EV, Cazzaniga RA, Aragão AF, Valois LQ, et al. Association between asthma, rhinitis and atopic dermatitis with leprosy: A case-control study. Indian J Dermatol Venereol Leprol 2023;89:834-41.
Abstract
Background
Considering the cross-regulation of Th1 and Th2 responses, we hypothesised that atopic diseases (Th2) inhibit the protective Th1 immune response to Mycobacterium leprae and exacerbates leprosy.
Objective
In this study, we aimed to evaluate the association between leprosy and atopic diseases.
Methods
To evaluate the association of atopic diseases with leprosy, we conducted a case-control study that included leprosy patients (n = 333) and their household contacts (n = 93). The questionnaire from the International Study of Asthma and Allergies in Childhood, which is validated in several countries for epidemiological diagnosis of atopic diseases, was applied to determine the occurrence of atopic diseases, allergic rhinitis, asthma, and atopic dermatitis among leprosy patients and the household contacts.
Results
Considering clinical and epidemiological data, among the leprosy group 51.6% (n = 172) were determined to have at least one atopic disease, while atopy was observed less frequently at 40.86% among household contacts (n = 38). When two or more atopic diseases were assessed, the frequency was significantly higher among the leprosy patients than in the household contacts (21.9% vs. 11.8%; P-value = 0.03). Likewise, the frequency of asthma was significantly higher among leprosy patients (21%) than in the household contacts (10.8%; P-value = 0.02). Thus, our analyses revealed an association of atopic diseases with leprosy, with a significant linear increase in the occurrence of leprosy with an increase in the number of atopic diseases (P-value = 0.01).
Limitation
Due to the difficulties in recruiting household contacts that have prolonged contact with patients, but are not genetically related to the patient, the household contacts group is smaller than the leprosy patient group.
Conclusion
The data reveal an association between atopic diseases and leprosy outcomes. This knowledge could improve the treatment of leprosy patients with co-incident atopic diseases.
Keywords
Leprosy
atopic dermatitis
allergic rhinitis
asthma
Plain Language Summary
Leprosy is a chronic and infectious diseases caused by the bacillus Mycobacterium leprae and affecting skin and peripheral nerves. The clinical presentation of leprosy is related to immune response profiles, with less severe clinical forms associated to Th1 response. Atopic disease is an inherited disorder, where the individual is predisposed to a predominant Th2 response to environmental proteins, manifested as rhinitis, asthma, and atopic dermatitis. Given the cross-regulation that occurs between Th1 and Th2 responses, we hypothesized that atopic diseases likely inhibit the immune response to M. leprae and exacerbate its presentation. To test this association, we examined records from leprosy patients and household’s contacts. The questionnaire from the International Study of Asthma and Allergies in Childhood (ISAAC), that is validated in several countries for epidemiological diagnosis of atopic diseases, was applied. Our analyses reveal that atopic diseases were associated with leprosy, with a significant linear increase in the occurrence of leprosy with an increase in the number of atopic diseases. This knowledge contributes to prompt in doctors the need of investigate leprosy in atopic diseases patients and positive epidemiology, helping the early diagnosis and treatment of these patients.
Introduction
Leprosy is a chronic infectious disease, caused by Mycobacterium leprae, and with 202,185 cases detected worldwide in 2019, remains an important global health concern.1 Brazil is among the countries with the highest prevalence of leprosy.2 Our recent study documented many cases of physical disability in leprosy patients less than 15 years of age, with cases among children indicating poor disease control.3
Since most individuals infected with Mycobacterium leprae do not develop leprosy, Mycobacterium leprae is considered only weakly pathogenic.4,5 In individuals in whom the disease does manifest, however, leprosy affects the skin and peripheral nerves and can cause deformities that dramatically impact the patient’s quality of life.4,5 For treatment purposes, leprosy is operationally classified as paucibacillary or multibacillary.5 Considering histopathological parameters, patients can be classified into one of the following clinical forms: indeterminate, tuberculoid, borderline and lepromatous leprosy.5-7 These presentations are closely associated with the host’s immune response, with protection against Mycobacterium leprae mediated by cellular immune responses characterised by T-helper 1 (Th1) and Th17 cells. Th1 responses are also associated with the paucibacillary forms where bacterial burdens are relatively low.5,8,9 In contrast, patients with the most severe clinical multibacillary presentations have higher infection levels and bacterial dissemination that are associated with predominantly Th2 and T-regulatory cells.5,6,8,10,11
Atopy is an immunological disorder with an inherited predisposition in which there are predominantly Th2-lymphocyte responses to small amounts of environmental proteins such as pollen, domestic dust mite and food allergens.12 Notably, the atopic diseases asthma, allergic rhinitis and atopic dermatitis share the same pathogenesis, are mediated by immunoglobulin E (IgE), and often appear together in the same individual or family.12,13 The cross-regulation that occurs between the Th1 and Th2 responses is now well described, with Th1-cells producing interferon-gama (IFN-γ) that can inhibit Th2 responses and, conversely, interleukin (IL)-4 produced by Th2-cells inhibiting antigen-specific Th1 responses.12,14,15 This interplay is most clearly demonstrated for helminth infections, such as schistosomiasis, where the Th2 responses are potent enough to interfere with the generation of effective responses following tetanus toxoid vaccination.16 It is widely believed that the reduced prevalence of autoimmune and atopic diseases in tropical developing countries is due to the widespread prevalence of infections (such as intracellular parasites etc.) that induce a Th2 and Treg response.16,17 Given this, some authors hypothesised that atopy, in which individuals have a predominance of the Th2 response12, can affect the Th1 profile that is required for protection against intracellular pathogens.5,8,11
Few studies have investigated the association between atopy and leprosy.18-20 Smith et al.19 found that 68% of leprosy patients also had an atopic disease, a rate higher than the 59% observed among controls. Another study showed higher concentrations of total IgE and specific-IgE for Mycobacterium leprae in leprosy patients than in the control group.20
We hypothesised that there would be an association between atopic diseases and leprosy, with atopic patients having a higher frequency of leprosy than their healthy contacts. Conversely, leprosy patients should have higher frequency of atopic diseases than household controls exposed to Mycobacterium leprae. To test this, we conducted a case-control study reviewing the medical records of leprosy patients and household contacts.
Methods
Study subjects
The study included 426 individuals divided into two groups: leprosy patients (n = 333) and the control group (n = 93), which was composed of genetically unrelated household contacts of leprosy patients [Figure 1]. However, some of them did not have complete clinical or demographic information in the medical records of the dermatology clinic, e.g lack of operational data, clinical classification, or birth date. The leprosy group consisted of a non-random sample that included all patients treated at the DESMANCHA clinic of the University Hospital (Federal University of Sergipe) and at the Centro de Especialidades Médicas de Aracaju that fulfilled the inclusion criteria. Leprosy was confirmed by biopsy and/or positive slit skin smear, and a clinical-epidemiological questionnaire was used for data collection of features such as bacilloscopy, leprosy reaction, dermato-neurological examination, and neurological disability. Leprosy patients were classified a) according to the operational classification into paucibacillary or multibacillary and b)according to the Ridley-Jopling classification7 as lepromatous leprosy; borderline lepromatous; borderline borderline; borderline tuberculoid; tuberculoid; indeterminate; pure neuritis leprosy.
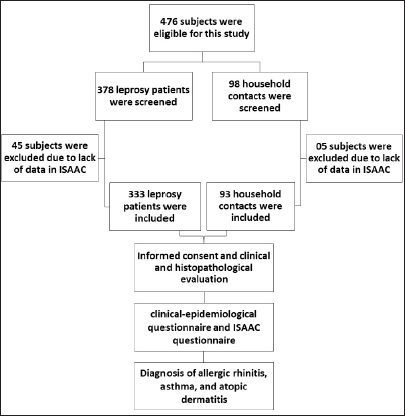
- Flowchart of the study population showing the number of leprosy patients and household contacts (HHC) screened, excluded and included in the study, and the study procedures
Each leprosy patient in the study was asked to bring unrelated household contacts and they were included within the control group. Controls were examined to exclude a diagnosis of leprosy. They were considered for inclusion if they had been resident in the same house as patients or had regular contact with them for more than five years. Household contacts were selected only if not having the same genetic inheritance.
Ethical approval
This project was conducted in accordance with the Helsinki Declaration and was approved by an ethics and research committee (CAAE-0152.0.107.000-07). Subjects voluntarily agreed to participate in the study and signed an informed consent form.
Study design
We conducted a case-control study, from January 2008 to December 2019, recruiting leprosy patients attending the dermatology clinic of the University Hospital. Their household contacts were also recruited with the approval of the index case and the household contacts. The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire, which has been validated in Brazil by several studies,21-24 was used to determine the occurrence of the following atopic diseases: allergic rhinitis, asthma, or atopic dermatitis. The diagnosis was considered if the patient had a positive answer to one of the following questions: (1) For rhinitis: have you ever had a runny nose or sneezing without having a cold? In the past 12 months have you had a runny nose or sneezing without having a cold? Or have you ever had allergic rhinitis? (2) For asthma: have you ever had wheezing in your life? In the past 12 months have you had wheezing? Or did you ever have asthma in your life? (3) For atopic dermatitis: have you ever had spots or itchy skin? In the last 12 months have you had these spots on your skin? Or have you ever had eczema? We defined an atopic patient as having had one or more of the following diagnoses: asthma, allergic rhinitis or atopic dermatitis. Atopy was further stratified according to the number of atopic diseases recorded for each participant (as either without atopic disease or with one, two or three atopic diseases). Herein, atopic features were considered the independent variable (exposure), and the occurrence of leprosy and its clinical forms were the dependent variables (the outcome).
Statistical analysis
Data were analysed using Microsoft Excel software - 2017. Quantitative variables were described as means and standard deviations. Student’s t-test was used to compare the differences in mean age between the groups. The outcome variable for each group was the the occurrence of leprosy cases) or not (household contacts). The chi-square test or Fisher’s exact test were used to assess statistical differences between groups, and significance level of P-value ≤0.05 and power equal to 0.80 were considered using IBM SPSS-Statistics/22.
Results
Clinical-epidemiological characteristics of the study participants
The clinical-epidemiological data of all study subjects is shown in Table 1. The mean age was 46.9 ± 17.2, and 52.8% (n = 225) of participants were female. As for education, 14.1% (60) were illiterate, 47.4% (202) had primary education and only 6.8% (29) had higher education. Additionally, 18.8% (80) had asthma; 36.6% (156) rhinitis; 17.4% (74) atopic dermatitis. In terms of frequency, 29.6% (126) had one atopic disease; 16% (68) had two, and 3.8% (16) had all three atopic diseases under investigation.
| Baseline characteristics | Total n = 426 % (n) | bCI 95% |
|---|---|---|
| Age (years) (Mean ± aSD) | 46.9 ± 17.2 | (range 8-88) |
| Gender | ||
| Male | 46.9 (200) | |
| Female | 52.8 (225) | |
| Groups | ||
| Leprosy | 78.2 (333) | 74.2-81.9 |
| Contact controls | 21.8 (93) | 18.1-25.8 |
| Atopic diseases | ||
| Allergic rhinitis | 36.6 (156) | 32.5-41.1 |
| Asthma | 18.8 (80) | 15.1-22.6 |
| Atopic dermatitis | 17.4 (74) | 14.1-20.9 |
| Non-atopic | 50.7 (216) | 45.9-55.2 |
| One atopic disease | 29.6 (126) | 25.2-34.2 |
| Two atopic diseases | 16 (68) | 12.6-19.5 |
| Three atopic diseases | 3.8 (16) | 2.0-5.6 |
aSD: Standard deviation, bCI: confidence interval
Comparison of clinical characteristics between leprosy and household contacts
The age, sex, race and education of leprosy and household contacts groups were similar, with no differences observed. Among the leprosy group, 35.35% (105) were operationally classified as paucibacillary, while 64.64% (192) were multibacillary. Further diagnostic scrutiny indicated that 24.4% (67) were lepromatous leprosy, 29.8% (82) tuberculoid, 11.3% (31) indeterminate and 6.2% (17) pure neural leprosy [Table 2].
| Characteristics | Leprosy (n = 333) |
Household contacts (n = 93) |
P-value |
|---|---|---|---|
| Age (years) (Mean ± aSD) | 46.7 ± 17.9 | 47.6 ± 14.6 | b 0.63 |
| Gender | % (n) | % (n) | |
| Male | 49.7 (165) | 37.6 (35) | 0.39 |
| Female | 50.3 (167) | 62.4 (58) | |
| Operational form | n = 297 | N/A | |
| Multibacillary (MB) | 64.64 (192) | N/A | |
| Paucibacillary (PB) | 35.35 (105) | N/A | |
| Clinical form | N = 275 | N/A | |
| Lepromatous leprosy (LL) | 24.4 (67) | N/A | |
| Borderline lepromatous (BL) | 4.4 (12) | N/A | |
| Borderline borderline (BB) | 18.2 (50) | N/A | |
| Borderline tuberculoid (BT) | 5.8 (16) | N/A | |
| Tuberculoid (TT) | 29.8 (82) | N/A | |
| Indeterminate leprosy (IL) | 11.3 (31) | N/A | |
| Pure neuritic leprosy (PNL) | 6.2 (17) | N/A | |
| Atopic diseases | |||
| Two or more atopic diseases | 21.9 (73) | 11.8 (11) | c 0.038 |
| Rhinitis | 38.1 (127) | 31.2 (29) | 0.218 |
| Asthma | 21 (70) | 10.8 (10) | c 0.025 |
| Atopic dermatitis | 18.9 (63) | 11.8 (11) | 0.111 |
aSD: Standard deviation, bStudent’s t-test, cFisher’s exact test, N/A: not applicable
Among the leprosy patients, 51.6% (172) were atopic and there was no difference in the frequency of atopic diseases among the operational forms of leprosy (multibacillary and paucibacillary). We observed that 54.7% (105) of the multibacillary were atopic, while 46.6% (49) were atopic paucibacillary patients. Among household contacts, 40.86% (38) were atopic. While the frequency of asthma was significantly higher among leprosy patients (21%) than in the household contacts (10.8%; P-value = 0.025), the difference in frequency of rhinitis or atopic dermatitis was not statistically significant. When two or more atopic diseases were considered, the frequency was significantly higher in the leprosy group than in the household contacts (21.9% vs. 11.8%; P-value = 0.038).
Frequency of atopic diseases in the clinical forms of leprosy
Upon more stringent analysis, the highest frequencies of atopic dermatitis occurred in borderline lepromatous (45.5%) followed by borderline (32.7%), borderline tuberculoid (33.3%), lepromatous leprosy (16.7%), tuberculoid (12.5%) and indeterminate (13.3%), we identified an association between the clinical forms of leprosy and the occurrence of atopic dermatitis (P-value = 0.016), [Table 3].
| Clinical features | LL (n = 67) % (n) |
BL (n = 12) % (n) |
BB (n = 50) % (n) |
BT (n = 16) % (n) |
TT (n = 82) % (n) |
IL (n = 31) % (n) |
PNL (n = 17) % (n) |
a P-value |
|---|---|---|---|---|---|---|---|---|
| Allergic rhinitis | 34.8 (23) | 54.5 (6) | 46.9 (23) | 46.7 (7) | 35.0 (28) | 23.3 (7) | 47.1 (8) | 0.279 |
| Asthma | 7.6 (5) | 36.4 (4) | 20.4 (10) | 26.7 (4) | 25.0 (20) | 10.0 (3) | 23.5 (4) | 0.031 |
| Atopic dermatitis | 16.7 (11) | 45.5 (5) | 32.7 (16) | 33.3 (5) | 12.5 (10) | 13.3 (4) | 11.8 (2) | 0.016 |
| Number of atopic diseases | 0.058 | |||||||
| Non-atopic | 53.0 (35) | 18.2 (2) | 38.8 (19) | 33.3 (5) | 50.0 (40) | 66.7 (20) | 52.9 (9) | |
| One atopic disease | 36.4 (24) | 36.4 (4) | 30.6 (15) | 33.3 (5) | 31.3 (25) | 20.0 (6) | 17.6 (3) | |
| Two or more atopic diseases | 10.6 (7) | 45.5 (5) | 30.6 (15) | 33.3 (5) | 18.8 (15) | 13.3 (4) | 29.4 (5) |
aFisher’s exact test, LL: lepromatous leprosy, BL: borderline lepromatous, BB: borderline borderline, BT: borderline tuberculoid, TT: tuberculoid, IL: indeterminate leprosy, PNL: pure neuritis leprosy
Similarly, we also observed that the highest frequencies of asthma occurred in borderline lepromatous (36.4%) and borderline tuberculoid (26.7%) leprosy (P-value = 0.031). Although the highest frequency of rhinitis also occurred in borderline lepromatous (54.5%) and borderline borderline (46.9%), with the lowest frequency in the indeterminate form (23.3%), no significant difference in the frequency of rhinitis was observed between the Ridley-Jopling forms.
Occurrence of leprosy according to the number of atopic diseases
Non-atopic study participants had a leprosy occurrence of 74.5%, those with one atopic disease had a leprosy occurrence of 78.6%, and those with two atopic diseases had a leprosy occurrence of 85.3% and those with three atopic diseases had a leprosy occurrence of 93.8%. Although the number of atopic diseases and occurrence of leprosy showed no difference between the groups (P-value = 0.12), there was a significant linear increase in the occurrence of leprosy as the number of atopic diseases rises (P-value = 0.01; linear trend analysis = 5.77; Figure 2; Table 4).
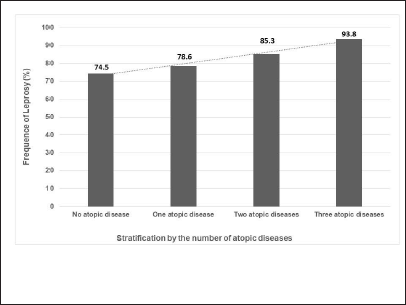
- Linear increase in the occurrence of leprosy according to the number of atopic diseases. Linear trend analysis = 5.773; P-value = 0.017
| Number of atopic diseases (n = 426) | Occurrence of leprosy % (n) | aCI 95% |
|---|---|---|
| 3 (n = 16) | 93.8 (15) | 81.3-100 |
| 2 (n = 68) | 85.3 (58) | 76.5-91.2 |
| 1 (n = 126) | 78.6 (99) | 71.4-84.1 |
| 0 (n = 216) | 74.5 (161) | 68.1-79.2 |
aCI: confidence interval, Fisher’s exact test
The clinical impact of the association of atopy and leprosy manifestation was evident in several study participants. As an example, Figure 3 shows photographs of a 10-year-old lepromatous leprosy patient. The patient had a history of asthma until she was 6 years of age with persistent symptoms of rhinitis and atopic dermatitis. Of note, the typical infiltration from leprosy can be observed on the ears and she had facial heterogeneous infiltrated lesions with redness and hypochromia. On her forearm and cubital fossae, abdomen, back of trunk and legs, there was an overlap of atopic dermatitis with scaly erythematous lichenified plaques and leprosy lesions with erythematous infiltrated papules.
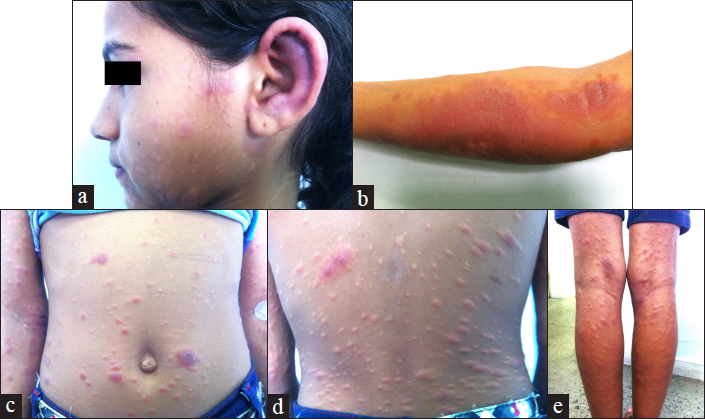
- Photographs from a 10-year-old girl with lepromatous leprosy disease. The patient had a history of asthma until 6 years old and has persistent symptoms of rhinitis and atopic dermatitis. (a) Her head showing the typical ear infiltration from leprosy and in her face, heterogeneous infiltrated lesions with redness and hypochromia. (b) forearm and cubital surface, (c) abdomen (d) back of trunk and (e) legs showing an overlap of atopic dermatitis with scaly erythematous lichenified plaques and leprosy lesions with erythematous infiltrated papules
Discussion
Mycobacterium leprae is a slow-growing bacillus that manifests as leprosy in a minority of infected individuals. In this study, we demonstrate an association between leprosy and atopic diseases. A linear increase in leprosy frequency was observed in patients presenting with an increase in the number of atopic diseases. It is known that atopic patients have predominantly Th2-responses that could negatively impact the generation of a protective Th1-response against Mycobacterium leprae. Thus, it is possible that atopic patients have a greater systemic imbalance in their immunity that results in a reduced ability to generate a protective response to Mycobacterium leprae, thereby leaving them prone to developing leprosy.
Asthma is an important cause of disability and impairs the quality of life.25 Similarly, leprosy remains a serious public health problem in Brazil, where the elimination level proposed by the WHO has not been attained.2,3 As individuals’ genetics predisposes them to develop a Th2-biased immune response and atopic diseases, we hypothesised that atopic diseases would also be associated with increased frequency of leprosy. Previous studies have shown that patients with schistosomiasis, a helminth infection that induces a potent Th2-response, have a compromised Th1-response in response to vaccination by tetanus toxoid.16 Studies in experimental models, and in humans, have conversely shown that infection by parasites associated with a Th1-profile can afford protection against autoimmune diseases.26-28
There are few studies associating atopic diseases and leprosy.18,19 Smith et al.19 reported that although the prevalence of atopic diseases was observed in 68% of leprosy patients relative to 59% among controls this difference was not statistically significant; moreover, neither the number of atopic diseases nor the severity of these diseases was assessed. Further, that study included fewer patients and the control group was comprised of volunteers from the hospital rather than household contacts. Thereby, this may have introduced a selection bias of subjects with atopic diseases and, unlike in our study where patients and controls were derived from the same households, any differences may have been masked by environmental variables.
The use of International Study of Asthma and Allergies in Childhood as a single and standardised instrument has allowed the generation of consistent data relating to the prevalence of rhinitis, asthma and atopic dermatitis among children and adolescents in Brazil and worldwide.29 Using International Study of Asthma and Allergies in Childhood, our study revealed 36.6% with rhinitis, 18.8% with asthma and 17.4% with atopic dermatitis. In Brazil, International Study of Asthma and Allergies in Childhood found an average prevalence in adolescents of 29.6% with rhinitis, 19% with asthma and 5% with atopic dermatitis. In Aracaju, International Study of Asthma and Allergies in Childhood revealed a prevalence of rhinitis of 19.9%, asthma of 16.5%, and atopic dermatitis of 11.4% in children aged 6-7 years. In adolescents, Aracaju had a prevalence of rhinitis of 25.6%, asthma of 18.7% and atopic dermatitis of 7.9%.24,29 Unfortunately, in the adult population comparable epidemiological information is not yet available, and only limited data are available with regard to the prevalence of allergic diseases.30 Furthermore, there is no single-standardised method for diagnosing atopic diseases in adults, making it exceedingly difficult to obtain an accurate prevalence of these diseases.31 Therefore, since leprosy is more common in adults due to its typically long incubation time, an important limitation of the present study is that our sample of 426 patients is composed mostly of adults. As most atopic diseases go into remission in adulthood, the prevalence rate in adults could be less, if only the active disease is considered. Although the International Study of Asthma and Allergies in Childhood questionnaire was designed for children and adolescents, some argue that it is possible to also use it for adults.30,32,33 In agreement, our data on the frequency of rhinitis and asthma are similar to that found in the population of adolescents by the International Study of Asthma and Allergies in Childhood epidemiological survey, in earlier studies.29,34 In a study among Iranian adults, Shoormasti et al.31 reported a prevalence of allergic diseases in 36.3%; 28.3% of rhinitis, 12.5% of asthma and 3.9% of atopic dermatitis, similar to the frequencies among the control group in our study.
In our study, the frequency of asthma was significantly greater among leprosy patients than in household contacts. Although the frequency of atopic dermatitis in household contacts (11.8%) was lower than that observed among leprosy patients (18.9%), this was not statistically significant. It is noteworthy that the detection of atopic dermatitis in adults can be challenging since approximately 95% of atopic dermatitis cases begin before five years of age and only a small fraction of patients continues to be affected by the disease as adults35. In a systematic review, Mathiesen and Thomsen35 identified that studies often used different strategies for the diagnosis of atopic dermatitis, including questionnaires, clinical examination, and telephone and video interviews. Likewise, different diagnostic criteria were used in the different studies: Schultz Larsen Criteria, Hanifin and Rajka criteria, International Study of Asthma and Allergies in Childhood, UK Working Party diagnostic criteria, GALEN (Global Allergy and Asthma European Network) and the patient’s report of the diagnosis given by a doctor. Among the 14 studies in 17 countries, the reported prevalence of atopic dermatitis ranged from 1.6 to 11.5 in the point prevalence or from 2.2 to 17.6% in one-year prevalence. The most common diagnostic criteria used were those of UK Working Party and Hanifin and Rajka.35 Studies that used the International Study of Asthma and Allergies in Childhood method in Thailand found a prevalence of 15% of one-year prevalence. These data are similar to the frequency found in our control group. The diagnostic criteria used in our study were positivity of any of the three criteria: having symptoms of rhinitis, asthma or atopic dermatitis at any time in life, having them in the last 12 months, or having a confirmed diagnosis of these diseases. The main argument for this definition is the knowledge that atopic diseases are genetically inherited, and therefore persistent in these patients. These criteria are validated by the concordance of the data of the control group with other studies from the literature. In this way, it is possible that the frequency of atopic patients in other studies has been less since mild cases of the disease may go unnoticed and many adult patients would be in the remission phase of the disease and, therefore, would not remember episodes that occurred in childhood.36,37 Although the clinical symptoms of atopic diseases may be less apparent in adulthood, there is a possibility that they had already influenced, and interfered with, the ability of the immune response to protect against intracellular infections. Considering that, complementary studies assessing the immune response should be conducted to formally test this hypothesis.
Although our study included 426 subjects, group sizes were uneven and we had a smaller number of controls than patients. This discrepancy arose due to the difficulties in recruiting household contacts that had prolonged contact with leprosy patients. We believe that the unwillingness of patients to reveal their diagnosis, due to the stigma of leprosy and other factors, along with this restriction on household contacts of lacking a degree of kinship, were factors that reduced the overall recruitment number of household contacts. Genetic and environmental factors can influence the susceptibility to leprosy. Our rigidity in household contacts selection allowed us to control for social and environmental factors as well as Mycobacterium leprae strengthens the quality of the observations we were able to make.
Conclusion
Collectively, our data indicate an association between atopic diseases and leprosy, particularly for asthma and the presence of two or more atopic diseases. A linear increase in the frequency of leprosy was associated with an increasing number of atopic diseases.
Acknowledgements
We thank the study participants, as well as the dermatologists and the nurses of the Leprosy Clinic of the University Hospital who helped in the treatment and follow-up of the patients included in this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This project was supported by Grants MCTIC/CNPq N 28/2018, Process: 421060/2018-2 and CHAMADA MS/CNPq/FAPITEC/SE/SES Nº 06/2018 - PPSUS SERGIPE 2017/2018. RAC was a postdoc supported by CAPES. ARJ and RPA are Scientists supported by CNPQ.
Conflict of interest
There are no conflicts of interest.
References
- Global leprosy (Hansen disease) update, 2019: Time to step-up prevention initiatives. 2019. Wkly Epidemiol Rec [Internet]. 92:501-20. Available from: http://apps.who.int/iris/bitstream/10665/254907/1/9789290225492-eng.pdf
- [Google Scholar]
- Leprosy in Brazil in the 21st century: Analysis of epidemiological and operational indicators using inflection point regression. An Bras Dermatol. 2020;95:743-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and epidemiological indicators and spatial analysis of leprosy cases in patients under 15 years old in an endemic area of Northeast Brazil: An ecological and time series study. BMJ Open. 2019;9:e023420.
- [CrossRef] [PubMed] [Google Scholar]
- Prevention of transmission of leprosy: The current scenario. Indian J Dermatol Venereol Leprol. 2020;86:115-23.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of innate and adaptative immune responses on the differential clinical outcomes of leprosy. Infect Dis Poverty. 2017;6:5.
- [CrossRef] [PubMed] [Google Scholar]
- The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-81.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-73.
- [PubMed] [Google Scholar]
- Distinct roles of Th17 and Th1 cells in inflammatory responses associated with the presentation of paucibacillary leprosy and leprosy reactions. Scand J Immunol. 2017;86:40-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine gene polymorphisms in type I and type II reactions in Hansen’s disease. Indian J Dermatol Venereol Leprol. 2020;86:482-8.
- [CrossRef] [PubMed] [Google Scholar]
- Increased serum levels of interleukin - 6 in erythema nodosum leprosum suggest its use as a biomarker. Indian J Dermatol Venereol Leprol. 2021;87:190-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mycobacterium leprae recombinant antigen induces high expression of multifunction T lymphocytes and is promising as a specific vaccine for leprosy. Front Immunol. 2018;9:2920.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers for severe asthma: Lessons from longitudinal cohort studies. Allergy Asthma Immunol Res. 2021;13:375-89.
- [CrossRef] [PubMed] [Google Scholar]
- Atopic diseases of childhood. Curr Opin Pediatr. 2002;14:634-46.
- [CrossRef] [PubMed] [Google Scholar]
- Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991;88:346-50.
- [CrossRef] [PubMed] [Google Scholar]
- The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;31:77-84.
- [CrossRef] [PubMed] [Google Scholar]
- Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269-72.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine profile associated with human chronic schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 2004;99:21-6.
- [CrossRef] [PubMed] [Google Scholar]
- Differential representations of memory T cell subsets are characteristic of polarized immunity in leprosy and atopic diseases. Int Immunol. 1999;11:1801-10.
- [CrossRef] [PubMed] [Google Scholar]
- Atopy and IgE in patients with leprosy. J Allergy Clin Immunol. 1990;85:795-800.
- [CrossRef] [PubMed] [Google Scholar]
- Total and anti-mycobacterial IgE levels in serum from patients with tuberculosis and leprosy. Tubercle. 1989;70:273-9.
- [CrossRef] [PubMed] [Google Scholar]
- International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire: Validation of the asthma component among Brazilian children. J Investig Allergol Clin Immunol. 1998;8:376-82.
- [PubMed] [Google Scholar]
- International Study of Asthma and Allergies in Childhood: Validation of the rhinitis symptom questionnaire and prevalence of rhinitis in schoolchildren in São Paulo, Brazil. Pediatr Allergy Immunol. 2001;12:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- International study of asthma and allergies in childhood (ISAAC): Validation of the written questionnaire (eczema component) and prevalence of atopic eczema among Brazilian children. J Investig Allergol Clin Immunol. 2002;12:34-41.
- [PubMed] [Google Scholar]
- Prevalência de sintomas de asma, rinite e eczema atópico entre crianças e adolescentes brasileiros identificados pelo International Study of Asthma and Allergies (ISAAC) - Fase 3. J Pediatr. 2006;82:4-10.
- [CrossRef] [Google Scholar]
- Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204.
- [CrossRef] [PubMed] [Google Scholar]
- Schistosoma mansoni infection modulates the immune response against allergic and auto-immune diseases. Mem Inst Oswaldo Cruz. 2004;99:27-32.
- [CrossRef] [PubMed] [Google Scholar]
- Role of pathogens in multiple sclerosis. Int Rev Immunol. 2014;33:266-83.
- [CrossRef] [PubMed] [Google Scholar]
- Parasites alter the pathological phenotype of lupus nephritis. Autoimmunity. 2014;47:538-47.
- [CrossRef] [PubMed] [Google Scholar]
- Asthma in children and adolescents in Brazil: Contribution of the International Study of Asthma and Allergies in Childhood (ISAAC) Rev Paul Pediatr. 2014;32:114-25.
- [CrossRef] [PubMed] [Google Scholar]
- Determining the score and cut-off point that would identify asthmatic adults in epidemiological studies using the asthma module of the International Study of Asthma and Allergies in Childhood questionnaire. J Bras Pneumol. 2005;31:477-85.
- [CrossRef] [Google Scholar]
- The prevalence of allergic rhinitis, allergic conjunctivitis, atopic dermatitis and asthma among adults of Tehran. Iran J Public Heal. 2018;47:1749-55.
- [PubMed] [Google Scholar]
- Prevalence of asthma and allergic rhinitis among adults in Yaounde, Cameroon. PLoS One. 2015;10:e0123099.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of asthma, allergic rhinitis and eczema among university students in Bangkok. Respir Med. 2002;96:34-8.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologia da asma: Estudo ISAAC (International Study of Asthma and Allergies in Childhood) Rev Bras Alerg e Imunopatol. 1998;21:38-45.
- [CrossRef] [Google Scholar]
- The prevalence of atopic dermatitis in adults: Systematic review on population studies. Dermatol Online J. 2019;25:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- The prevalence of atopic dermatitis, asthma, and allergic rhinitis and the comorbidity of allergic diseases in children. Environ Health Toxicol. 2012;27:e2012006.
- [CrossRef] [PubMed] [Google Scholar]
- Japanese guidelines for atopic dermatitis. Allergology Int. 2017;66:230-47.
- [CrossRef] [PubMed] [Google Scholar]






