Translate this page into:
Amivantamab (JNJ-61186372)-induced adverse cutaneous reaction
Corresponding author: Bing-Jun Shi, Department of Dermatology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing First People’s Hospital, Chongqing, China. shbingj@126.com
-
Received: ,
Accepted: ,
How to cite this article: Cheng J-R, Hui H-Z, Zheng J, Mao H, Wang YJ, Shi B-J. Amivantamab (JNJ-61186372)-induced adverse cutaneous reaction. Indian J Dermatol Venereol Leprol. 2024;90:108-10. doi: 10.25259/IJDVL_717_2022
Sir,
The Food and Drug Administration (FDA) granted accelerated approval to amivantamab for adult patients with locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, whose disease has progressed on or after platinum-based chemotherapy. Activating EGFR mutations render resistance to treatment with EGFR tyrosine kinase inhibitors. This resistance is mediated by multiple mechanisms, which include secondary EGFR mutation and activation of the c-mesenchymal-epithelial transition factor pathway. Amivantamab is a bispecific monoclonal antibody that targets both EGFR and mesenchymal-epithelial transition factor. 1 Acting on both targets provides amivantamab an “avidity effect,” which is superior to poziotinib or cetuximab in terms of therapeutic efficacy. 2 We report a case of adverse cutaneous reaction following amivantamab treatment.
A 53-year-old man without a history of skin disease was diagnosed as right lung adenocarcinoma with multiple mediastinal lymph node metastases. His genetic testing showed epidermal growth factor receptor (EGFR) exon 20 insertion mutations and hence was treated with amivantamab (JNJ61186372)—targeted therapy. The dosage of amivantamab was 1050 mg weekly for the first four weeks, and then every two weeks thereafter. He began to develop facial skin lesions that gradually extended to the scalp, buttocks and lower limbs after three weeks of treatment. He complained of skin pain but without any accompanying constitutional symptoms like fever, breathlessness, facial oedema, jaundice, etc. Dermatological examination showed purulent crusts on the scalp, [Figure 1] scattered erythematous papules on the face, legs and scrotum along with scattered crusts on the face [Figures 2–4]. Erosive erythema was seen on the perianal area [Figure 5]. Nails showed paronychia-like changes [Figures 6]. The cutaneous adverse events were grade 3 in severity. The pulse, blood, pressure, respiratory rate, temperature, lymph nodes were normal. Scalp pus culture showed S. aureus infection, with sensitivity to amoxicillin, gentamicin and tetracycline. His haemogram, renal and liver function tests, absolute eosinophil count and peripheral smear analysis were within normal limits. He was treated with kangfuxin liquid, compound polymyxin b ointment, compound neomycin ointment and recombinant human epidermal growth factor gel without systemic steroids and continued amivantamab treatment. Skin lesions improved after a week. He received 22 more injections of amivantamab, and the rash appeared basically similar to the initial manifestation after about a week of each infusion. In subsequent recurrences, the pus culture and sensitivity did not reveal any pathogens and each time, the skin lesions responded to recombinant human epidermal growth factor gel, compound polymyxin b ointment and kangfuxin liquid. His scoring was six based on the Naranjo adverse drug reaction probability scale, indicating a probable drug reaction to amivantamab.

- Erosions and purulent crusts on the scalp.
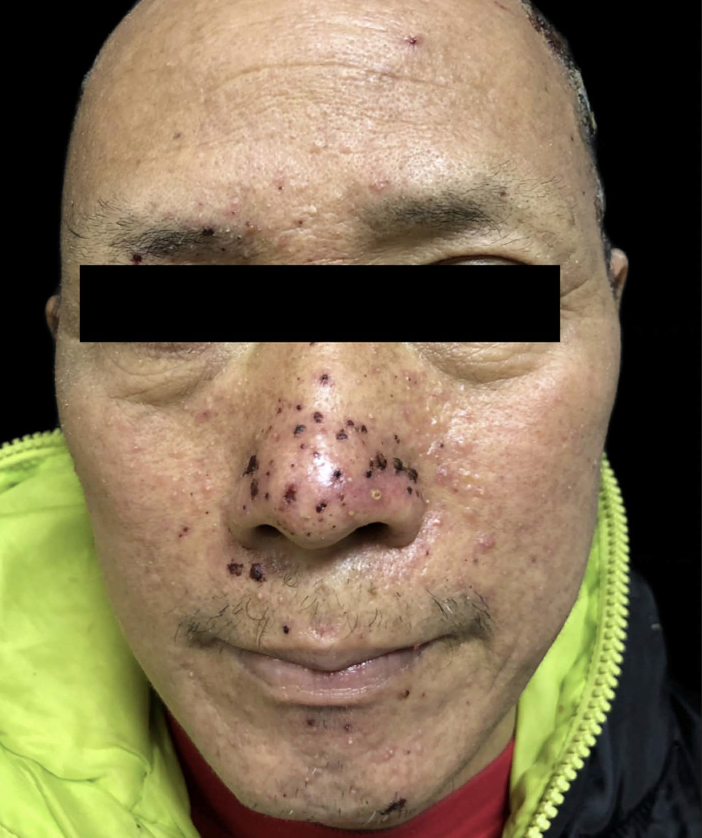
- Scattered erythematous papules and crusts on the face.
Amivantamab (30% Relative Risk) is safer and controllable compared with poziotinib, and only mild keratosis appeared on the face in amivantamab-treated BA/F3-bearing NOG mice models.2 Amivantamab has shown no cutaneous adverse effects in trials with cynomolgus monkeys.3 However, during the first infusion, skin lesions were the most common adverse reaction, and nine percent patients had grade 3 severity.4 In the CHRYSALIS phase I study, Park et al. documented adverse events like acneiform eruptions (86%), paronychia (45%), stomatitis (21%), pruritus (17%), diarrhoea (12%), pneumonitis (4%), hypoalbuminemia (27%), peripheral oedema (27%), etc.5 Nonetheless, amivantamab heralds a new era in the treatment of non-small cell lung cancer, but understanding the cutaneous side effects is also important. Kangfuxin liquid is made from American plant extract that is rich in active substances, and in combination with compound polymyxin b ointment and recombinant human epidermal growth factor gel reduces inflammation and promotes epidermal cell growth.6
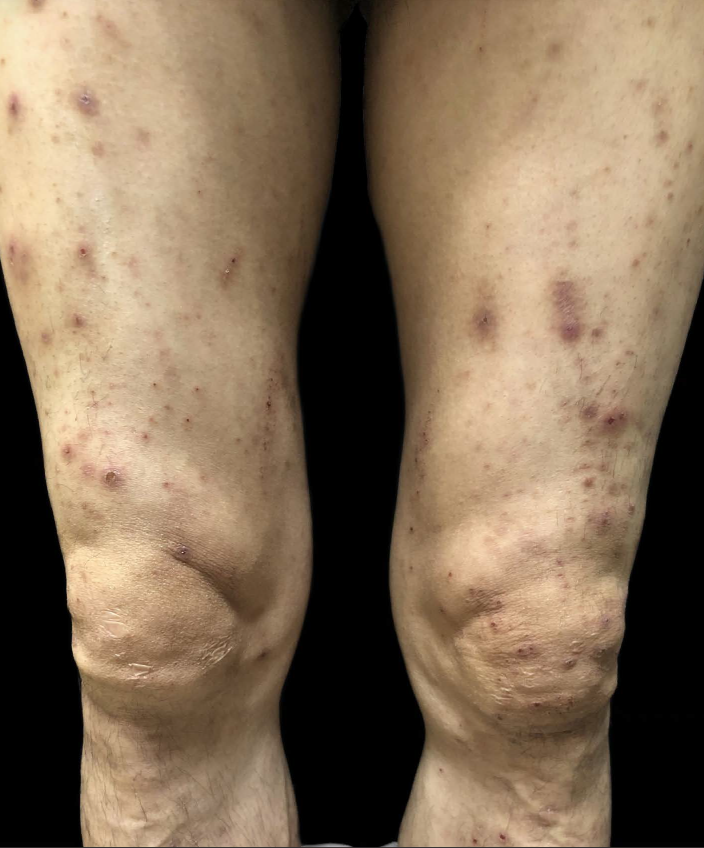
- Scattered erythematous papules on legs.

- Scattered erythematous papules on scrotum (red arrows).

- Erosive erythema on the perianal area.

- Paronychia-like changes (red arrows).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Antitumor activity of amivantamab (JNJ–61186372), an EGFR–MET bispecific antibody, in diverse models of EGFR exon 20 insertion–driven NSCLC. Cancer Discov. 2020;10:1194-209.
- [CrossRef] [PubMed] [Google Scholar]
- A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76:3942-53.
- [CrossRef] [PubMed] [Google Scholar]
- EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev. 2020;90:102105.
- [CrossRef] [PubMed] [Google Scholar]
- Amivantamab in EGFR exon 20 insertion–mutated non–small–cell lung cancer progressing on platinum chemotherapy: Initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39:3391-402.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of the effect of Kangfuxin liquid on diabetic patients with skin ulcers. Evid Based Complement Alternat Med. 2021;2021:1334255.
- [CrossRef] [PubMed] [Google Scholar]





