Translate this page into:
JAK-STAT inhibitors in Immune mediated diseases: An Overview
Corresponding author: Dr. Siba P. Raychaudhuri, Department of Veterans Affairs, 1VA Sacramento Medical Center, Northern California Health Care System, California, United States. siba.raychaudhuri@ucdmc.ucdavis.edu
-
Received: ,
Accepted: ,
How to cite this article: Shah RJ, Banerjee S, Raychaudhuri S, Raychaudhuri SP. JAK-STAT inhibitors in Immune mediated diseases: An Overview. Indian J Dermatol Venereol Leprol 2023;89:691-9
Abstract
For any biological response, transmission of extracellular signals to the nucleus is required for DNA transcription and gene expression. In that respect, cytokines/chemokines are well-known inflammatory agents which play a critical role in signalling pathways by activating the Janus kinase–signal transducers and activators of transcription (JAK-STAT) signalling proteins (Janus kinase–signal transducers and activators of transcription) which are a group of intracellular kinase molecules. Cytokines are a category of small proteins (∼5–25 kDa) that play a major role in cell signalling and are major drivers of an autoimmune response. Here we will discuss the role of Janus kinase–signal transducers and activators of transcription kinase cascades in the inflammatory-proliferative cascades of autoimmune disease and about the recent progress in the development of oral synthetic Janus kinase inhibitors (JAKi) and their therapeutic efficacies in dermatologic and systemic autoimmune diseases.
Therapeutic efficacy of Janus kinase inhibitors is now well established in the treatment of array of autoimmune and inflammatory disease: spondylarthritis with a special focus on psoriatic arthritis (PsA) and its dermatologic manifestations (psoriasis) and ankylosing spondylitis (AS), atopic dermatitis (AD), alopecia areata (AA), rheumatoid arthritis (RA) and inflammatory bowel disease (IBD).
In addition to the first-generation Janus kinase inhibitors several new-generation Janus kinase inhibitors are currently being evaluated. It is expected that these Janus kinase inhibitors likely have higher potency and less adverse effects as compared to their predecessors. Here we have discussed: (1) the functional significance of the Janus kinase–signal transducers and activators of transcription kinase cascades in the inflammatory-proliferative processes of autoimmune diseases and its cellular/molecular mechanisms and (2) progress in the development of oral synthetic Janus kinase inhibitors and their therapeutic efficacies in several systemic and cutaneous autoimmune diseases.
Keywords
Signalling molecules
JAK-STAT
autoimmune skin disease
therapy
Introduction
The clinical manifestations of autoimmune diseases vary depending on genetic predisposition and immune response with respect to the T cell subpopulations along with their associated inflammatory cytokines. Recent evidence suggests that the cytokine-induced Janus kinase signalling system plays an important role in the pathogenesis of an array of autoimmune/inflammatory diseases. Psoriasis is the first skin condition for which JAK-STAT-directed therapies were evaluated along with their associated conditions, the spondyloarthropathy which is a heterogeneous group of immune-mediated inflammatory conditions. Here we will discuss the evidence and significance of the intracellular Janus kinase signalling pathway in the inflammatory/proliferative cascades of autoimmune diseases and opportunities for therapeutic intervention through inhibition of this pathway. 1–3
Inflammation of connective tissue many a times is the result of coordinated interactions between the APC (antigen-presenting cells) and the TCR (T-lymphocyte receptor). 1–5 Subsequently, this T cell activation induces a local inflammatory response resulting in tissue damage through release of various pro-inflammatory chemokines/cytokines and activation of inflammatory cells with innate functions such as monocytes, macrophages and granulocytes 1,3,6–8
Various extracellular cytokines and growth factors transmit signals to the nucleus through the Janus kinase- signal transducers and activators of transcription signalling pathway. Cytokines/growth factors along with their receptor induce DNA transcription and gene expression through several steps [Figure 1]. The first step for signal transduction includes the interaction of cytokines with the cell surface receptors to induce conformational changes. Next, cytoplasmic kinase proteins are phosphorylated, resulting in phosphorylation and dimerization of signal transducers and activators of transcription (STAT) molecules which leads to intracellular migration of the STAT molecule which functions as a transcription factor for DNA transcription and activates gene expression [Figure 1]. This family of intracellular tyrosine kinase molecules known as Janus kinases are associated with the signalling pathways of multiple pro-inflammatory cytokines as shown in Figure 2 which are associated with the induction of inflammation 9,10
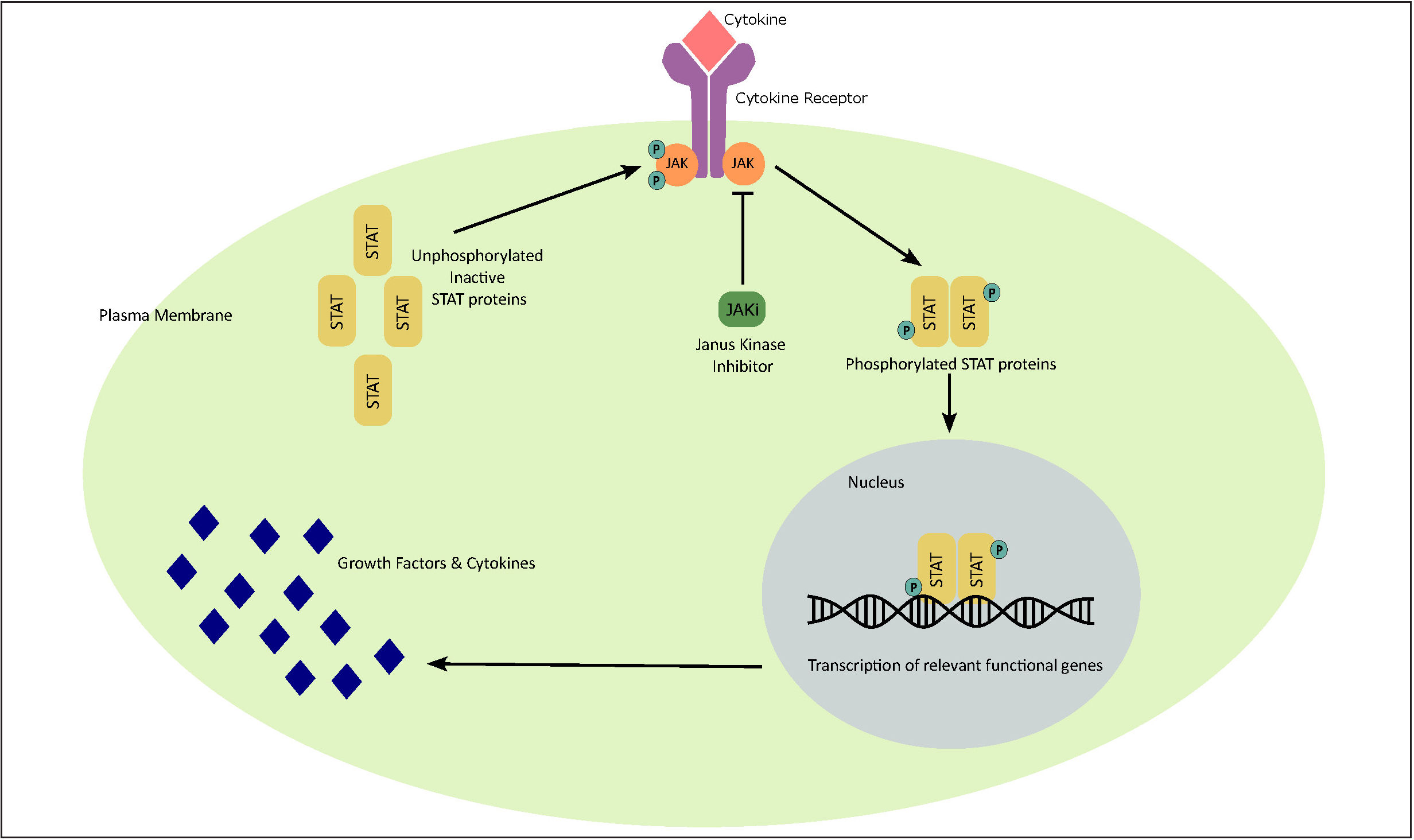
- Janus kinase–signal transducers and activators of transcription (JAK-STAT) signalling pathway. Interaction of cytokine with the respective cellular surface receptor leads to conformational changes in its intracellular domain and subsequently causes phosphorylation of intracellular Janus kinase (JAK) proteins. Phosphorylated Janus kinases lead to activation, phosphorylation and dimerization of signal transducers and activators of transcription (STATs) which then migrate as homo/hetero dimers into the nucleus and bind to specific DNA binding sites. This induces gene transcription and production of various cytokines involved in various inflammatory immune responses/functions as illustrated in Figure 2.
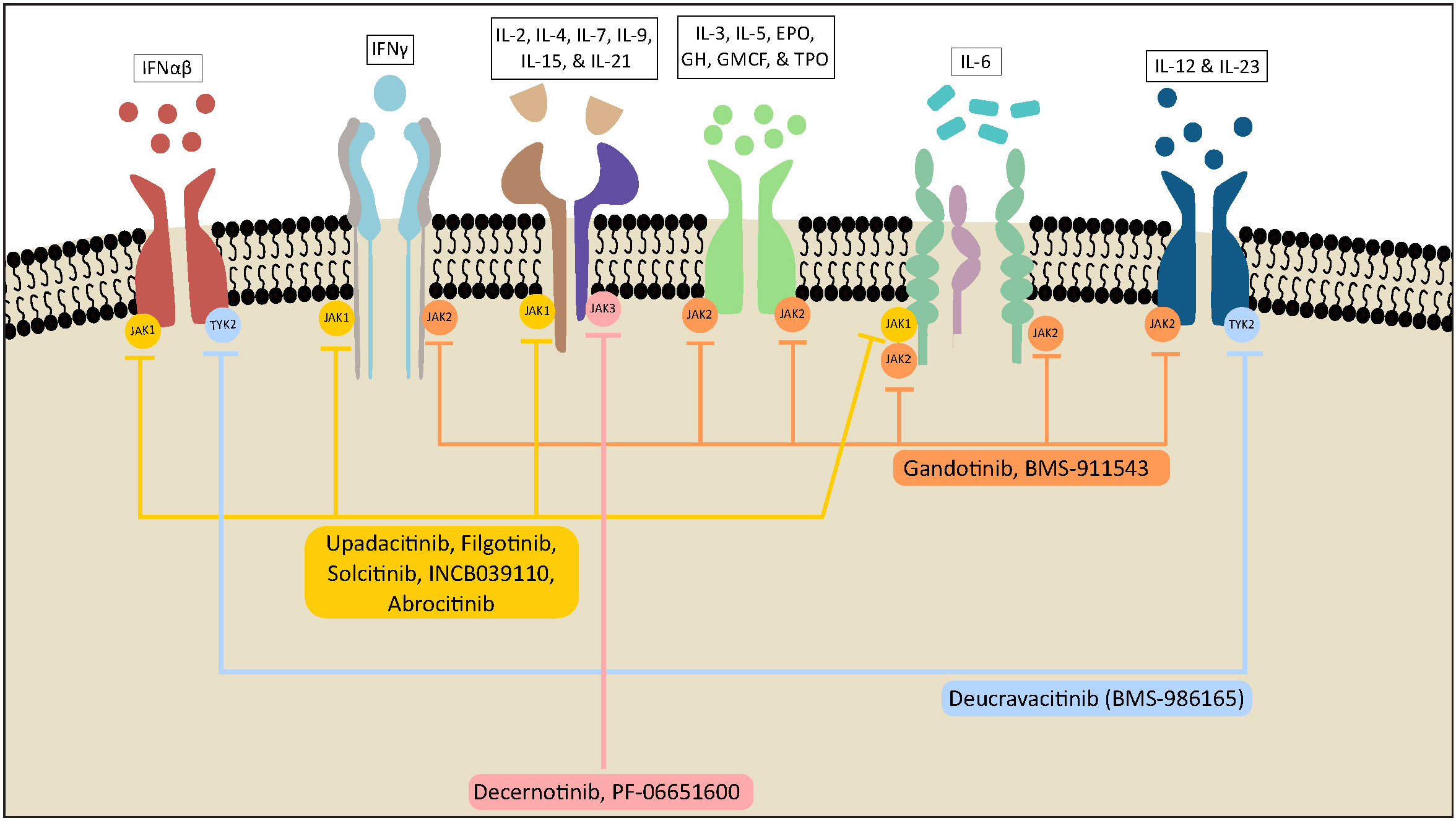
- Role of Janus kinase–signal transducers and activators of transcription (JAK-STAT) signalling and mechanism of action of Janus kinase inhibitors in various autoimmune diseases. As illustrated in Figure 2, various inflammatory cytokines, including interferons, interleukins, interferon-like cytokines, growth factors and colony-stimulating factors, bind to their specific receptors resulting in the activation of specific JAK-STAT pathways. Specifically, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-12, IL-15, IL-21 and IL-23 participate in JAK STAT activation and lead to immune responses as discussed in Table 1 thus contributing to the pathophysiology of various autoimmune diseases. It also demonstrates how the newer Janus kinase inhibitors block specific Janus kinase molecules leading to targeted disease control.
With the broader understanding of Janus kinases on the potentiation of pro-inflammatory signals, a new field in clinical immunology has emerged to evaluate the role of Janus kinases in the pathogenesis of autoimmune disease and the possibilities for its therapeutic application 1–3,10–12 In this article, we will describe the functional significance of Janus kinase–signal transducers and activators of transcription kinase cascades in various cutaneous and systemic inflammatory diseases of autoimmune origin and clinical use of these novel topical and oral synthetic Janus kinase–signal transducers and activators of transcription kinase inhibitors for these conditions. 1–3,13–15
Janus kinase–signal transducers and activators of transcription Signalling Pathway
Role of Janus Kinase/Signal Transduction and Activator of Transcription (JAK-STAT) signalling pathway has been implicated in the pathogenesis of various immune-mediated diseases. Four Janus kinases (JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2)) and seven signal STATs (STAT 1, 2, 3, 4, 5, 5a and 6) are included in this family of JAK-STAT pathway. 1,3,9,10 As noted in the pathogenesis of several immune-mediated diseases, various cytokines such as interferons, interferon-like cytokines, growth factors/hormones as well as colony-stimulating factors bind to their cognate Type I/II cytokine receptors and stimulate the JAK-STAT pathway [Figure 2]. This leads to the activation of Janus kinase molecules associated with the intracellular component for these cytokine receptors followed by phosphorylation of STAT molecules which in turn transmit the signals into the nucleus. This leads to the transcription of various cytokine-specific genetic programmes and release of these inflammatory molecules [Figures 1 and 2]. 1,2,15
Role of the Janus Kinase–Signal Transducers and Activators of Transcription Signalling System in Various Immune-Mediated Diseases
Various cytokines as described in Table 1 are responsible for the oligomerization of cytokine receptors leading to activation of intracellular receptor–associated JAKs and their phosphorylation, subsequently leading to activation of STAT molecules which then translocate to the nucleus and stimulate the transcription of effector genes. As demonstrated in Figure 2, JAK-STAT regulatory pathway has been involved in the production of multiple cytokines and chemokines which impacts growth, apoptosis as well as activation of B and T lymphocytes suggesting its regulation in the pathogenesis of several autoimmune diseases.
1–3,9–17
Ligand
Receptor
Target JAK kinase
Functions
Interleukin-2
IL-2Rα+IL-2Rb+γc
JAK1,2,3
Promotes proliferation and differentiation of effector and memory cells and promotes regulatory T cell development
Interleukin-4
IL-4Rα+γcR or IL-4Rα+IL-13Rα1
JAK1,3
Promotes Th2 cell differentiation and proliferation. Noted to have a contributory role in various allergic conditions, atopic dermatitis and Churg–Strauss syndrome
Interleukin-5
IL-5R+βc
JAK2
Regulatory cytokine associated with Th2-induced inflammation
Interleukin-9
IL-9R
JAK1,3
Promotes survival and activation of T cells and also induces pannus formation
Interleukin-12
IL-12Rβ1+IL-12Rβ2
JAK2,TYK2
Induces Th1 cell differentiation
Interleukin-13
IL-13Rα1+ IL-13Rα2+ IL-4Rα
JAK1,2,TYK2
Regulatory cytokine associated with Th2-induced inflammation
Interleukin-22
IL-22Rα1 or α2 +IL-10R2
JAK1,TYK2
Contributes to inflammation by stimulating epithelial cell proliferation and production of cytokines and chemokines. Involved in the pathogenesis of psoriasis, psoriatic arthritis and ankylosing spondylitis
Interleukin-23
IL-12Rβ1 + IL-23R
JAK2,TYK2
Induces Th17 cell differentiation and expansion
Interferon gamma
IFN-gR1+IFN-γR
JAK1,2
A key cytokine from Th1 cells. Proinflammatory cytokine for rheumatoid arthritis and psoriatic disease.
As detailed in Table 1, JAK-STAT signaling pathway regulates functions of various T cell subpopulations and their signature cytokines which contribute to the immune-mediated inflammation in multiple autoimmune diseases including rheumatoid arthritis, psoriasis, psoriatic arthritis, ankylosing spondylitis, atopic dermatitis to name a few 1–3,5–10
A number of animal studies have reported the role of JAK-STAT signaling pathway in the pathogenesis of immune-mediated diseases. In one study, an oral lavage of Janus kinase inhibitors (JAKi) was administered twice daily and it was shown to suppress inflammation and inhibit formation of periosteal/entheseal bone. It has also reported statistically significant reduction (p 0.05) in the scores indicating histologic and clinical inflammation in the SKG mice after treatment with Janus kinase inhibitors. 18 Studies have reported how silencing RNA is likely to impact the function of JAK1-3 and TYK2 in CD4 T cells and hence inhibits the secretion of pro-inflammatory cytokines and impairs the function of a broad spectrum of cytokines such as type I IFN, IL-12 and IL-23 signalling, IL-22, IL-17A and IL-17 F. 19,20 A few single nucleotide polymorphisms (SNPs) have been noted to suppress TYK2 isoform which in turn inhibits the signalling pathway and cytokine production 19 Thus, these in vivo/ex vivo studies provide the foundations for the concept that impacting JAK-1,2,3 and TYK2 will lead to inhibitions of an array of pro-inflammatory cytokines/chemokines and will be a wonderful target for various immune-mediated diseases such as psoriasis, lupus, alopecia areata, psoriatic arthritis, ulcerative colitis and ankylosing spondylitis. 19,20 In a study by Gracey et al., NDI-031407A, a potent and specific TYK2 inhibitor, was noted to prevent progression of SpA in the mouse model. It was noted to selectively inhibit TYK2 which in turn leads to inhibition of IL-23 pathway which is essential in the pathogenesis of spondyloarthropathy. It was also noted to forestall the joint space narrowing and bone marrow oedema was also noted on MRI. 21 Model NDI-031407 also protected mice from enthesis-related synovitis and bone marrow oedema in the IL-23 mini-circle. The study also demonstrated that the loss of function of TKY2 SNP (rs12720356) was more frequently noted in subjects with ankylosing spondylitis (AS) with less severe disease as noted by lower rates of spinal fusion. Association between JAK-2 polymorphisms have been reported with ankylosing spondylitis, 22 signal transducers and activators of transcription 3 polymorphism with psoriasis 23 and Janus kinase–signal transducers and activators of transcription signalling system nucleotide polymorphism have been noted in Crohn’s disease. 24 Thus, various JAK-STAT kinase isoforms might be involved in the kinetics of the disease process of multiple cutaneous and systemic immune-mediated diseases.
Of all four isoforms of Janus kinases, JAK3 expression is mainly noted in haematopoietic/immune cells as compared to rest of the isoforms-JAK1, JAK2 and TYK2 expression noted ubiquitously in mammals. JAK3 isoforms are associated with the common gamma chain pathway which is involved in the production of various cytokines including Interleukins-2,4,7,9,15 and 21. These interleukins are required for the activation, proliferation as well as differentiation of T and B lymphocytes. Interestingly, tofacitinib was initially developed with the idea to inhibit JAK3 to develop novel therapies for autoimmune diseases; however, it was later noted to have effect on JAK1 and JAK2 as well, making it a non-selective pan-Janus kinase inhibitor. 23–26
In addition to immune cells, Janus kinase isoforms have been identified in certain non-immune cells including in fibroblast like synoviocyte (FLS), the joint synovial cells as well as in keratinocytes which may provide additional justifications for the role of Janus kinase inhibitors in the treatment of psoriasis, psoriatic arthritis (PsA) and rheumatoid arthritis. 25–28 Subsequent studies have indicated a possible role of Janus kinase–signal transducers and activators of transcription kinase cascade in the pannus formation and activation of synovial Th17 cells in patients with psoriatic arthritis. A co-culture study of FLS (synovial fibroblasts) in psoriatic arthritis patients showed reduced expression of phosphorylated Janus kinase isoforms on exposure to tofacitinib. It was noted that JAK1/JAK2 inhibition by tofacitinib in psoriatic arthritis explants was noted to inhibit the fibroblast like synoviocyte migration and resultant production of certain FLS chemokines. 29 One of our subsequent studies demonstrated the significance of IL-23 induced JAK2/STAT3 signalling pathway in the pathogenesis of immune-mediated diseases especially in psoriatic arthritis by demonstrating the impact of JAK2/STAT3 kinases on the activation and proliferation of the pathologic IL-17+ Effector Memory T (TEM) cells. 30 Tofacitinib has been noted to inhibit the IL-23-induced proliferation of these IL-17+ TEM cells 30 suggesting its role in the treatment of psoriatic arthritis.
Classification of Janus kinase inhibitors
JAK inhibitors are novel small molecular drugs that are categorized as targeted synthetic disease-modifying anti rheumatic drugs (ts-DMARDs). Compared to biologics which inhibit specific cytokines, Janus kinase inhibitors bind reversibly and irreversibly to the intracellular Janus kinase proteins and thus inhibit the signalling pathway of multiple cytokines as described in Table 1. This, in turn, leads to the inhibition of transcription of cytokine-induced genetic pathways which are involved in the pathogenesis of various immune-mediated diseases including autoimmune inflammatory arthritis, psoriasis, atopic dermatitis, alopecia areata, vitiligo amongst others.
Over the course of last few years, many Janus kinase inhibitors have been developed which differ in their selectivity for various Janus kinase isoforms. As discussed above, there are four main Janus kinase isoforms: JAK1, JAK2, JAK3 and TYK2. First-generation JAK inhibitors are non-specific and inhibit multiple Janus kinase isoforms while the newer generation JAK inhibitors are more selective and specific, making them more desirable, especially due to fewer side effects [Table 2].
JAK inhibitors
Target
Indications
Current Approval Status
First-generation Janus kinase inhibitors
Baricitinib
JAK1/JAK2
Rheumatoid arthritis, alopecia areata, COVID-19
FDA approved
Ruxolitinib
JAK1/JAK2
Atopic dermatitis (topical), polycythemia vera, myelofibrosis, graft versus host disease
FDA approved
Tofacitinib
JAK1/JAK3
Rheumatoid arthritis, psoriasis, psoriatic arthritis, inflammatory bowel disease
FDA approved
Next-generation Janus kinase inhibitors
Upadacitinib
JAK1> JAK2,JAK3
Rheumatoid arthritis, psoriatic arthritis, atopic dermatitis
FDA approved
Abrocitinib
JAK1
Moderate to severe atopic dermatitis
FDA approved
Pacritinib
JAK2> JAK3, TYK2
High-risk myelofibrosis
FDA approved
Fedratinib
JAK2
Myelofibrosis
FDA approved
Filgotinib GLPG0634
JAK1>JAK2
Rheumatoid arthritis (approved by EMA)
EMA approved
Peficitinib (ASP015K)
Pan-JAK
Rheumatoid arthritis (approved in Japan, Korea)
Approved in Japan, Korea
Decernotinib (VX-509)
JAK3
Rheumatoid arthritis
Currently under trial
Solcitinib (GSK2586184)
JAK1
Moderate to severe plaques psoriasis, moderate to severe ulcerative colitis
Currently under trial
Deucravacitinib (BMS-986165)
TYK2
Moderate to severe chronic plaque psoriasis
Currently under trial
Itacitinib (INCB039110)
JAK1>JAK2
Lymphoma
Recently completed phase III trial
Ochromycinone (STA-21)
STAT3
Topical drug in psoriasis
Completed phase II trial
PF-06700841
TYK2/JAK1
Moderate to severe plaque psoriasis
Completed phase IIa trial
PF-06651600
JAK3
Alopecia areata
Completed phase III trial
First-Generation Janus kinase inhibitors: Include ruxolitinib, tofacitinib and baricitinib.
-
Ruxolitinib: It was the first Janus kinase inhibitor approved by FDA for polycythaemia vera. It acts against JAK1 and JAK2 isoforms. 31
-
Tofacitinib: Tofacitinib was the first Janus kinase inhibitor approved by the FDA for autoimmune diseases. Being a first-generation Janus kinase inhibitor, it is non-selective and hence acts against JAK1 and JAK 3 isoforms along with some JAK2 inhibition but with negligible activity against TYK2. 15,32 Since all Janus kinase isoforms are inhibited, they effectively block several gamma chain cytokines including IL-2,4,15 and 21. 33
-
Baricitinib: Baricitinib was noted to reversibly inhibit JAK1 and JAK2 isoforms with modest activity against JAK3 and TYK2. 34,35
-
Peficitinib: Pan Janus kinase inhibitor approved for use in Japan and Korea.
Next-generation Janus kinase inhibitors
Compared to first-generation Janus kinase inhibitors, these are more selective and act specifically against one Janus kinase isoform. Given that the first-generation Janus kinase inhibitors were multiple Janus kinase inhibitors, there could be more adverse effects such as bone marrow suppression leading to pancytopenia which leads to the principle of development of selective Janus kinase inhibitors inhibiting specific isoforms including JAK1, JAK2, JAK3 and TYK2. The next-generation Janus kinase inhibitors are expected to help treat specific inflammatory disorders with fewer side effects. With the technological advances in the field, there are now several next-generation Janus kinase inhibitors in the pipeline with the goal to have high efficacy small molecules with minimal off target effects [Table 2]:
-
Upadacitinib: It was one of the first newer generation Janus kinase inhibitors developed selectively against JAK1 isoforms.
-
Figlotinib: Highly selective JAK1 inhibitor approved for use in Europe and Japan for rheumatoid arthritis.
Role of Janus kinase Inhibitors in the treatment of various immune-mediated diseases
In this section, we will briefly outline the clinical uses of Janus kinase inhibitors in various immune-mediated diseases as approved by regulatory bodies including FDA and EMA.
Ruxolitinib
Ruxolitinib has been approved by FDA for myeloproliferative disorders like polycythemia vera and myelofibrosis and graft versus host disease 33 Topical ruxolitinib is approved for atopic dermatitis and segmental vitiligo. Further studies are still warranted to demonstrate the use of ruxolitinib in other autoimmune diseases.
Tofacitinib
Given the wide array of cytokines it inhibits, tofacitinib has been noted to be effective in several autoimmune diseases and has been approved by FDA for spondylarthritis (psoriatic arthritis and ankylosing spondylitis), rheumatoid arthritis and inflammatory bowel disease.
Baricitinib
Baricitinib has been approved by FDA as well as EMA for patients with rheumatoid arthritis and has shown good efficacy in Phase II trials against psoriasis, atopic dermatitis and systemic lupus erythematosus. 36 While it was also approved for use in COVID-19 patients requiring supplemental oxygen therapy, most recently, baricitinib became the first systemic treatment for alopecia areata approved by FDA in June 2022.
Upadacitinib
Upadacitinib has been approved by FDA for rheumatoid arthritis, psoriatic arthritis as well as atopic dermatitis.
Baseline investigations, monitoring guidelines and contraindications. Live or live-attenuated vaccines should generally not be administered during treatment:
Baseline investigations to be obtained prior to initiation of Janus kinase inhibitors:
-
Complete blood count with differential
-
Liver function panel including alanine transaminase, aspartate transaminase, bilirubin and alkaline phosphatase
-
Renal function for dose adjustment if needed
-
Lipid panel including total cholesterol, triglycerides, LDL and HDL
-
QuantiFERON test to evaluate for latent tuberculosis
-
Screening for latent tuberculosis, hepatitis B (including testing for hepatitis B virus [HBV] surface antigen and HBV core antibody), and hepatitis C virus (HCV) antibody.
-
Pregnancy testing in women of reproductive age group
-
Complete blood count: 4–8 weeks after initiation of treatment followed by every 3 months thereafter
-
Liver function panel: 4–8 weeks after initiation of treatment followed by every 3 months thereafter
-
Lipid panel: 4–8 weeks after initiation of treatment
-
History of drug-induced hypersensitivity reaction
-
Severe hepatic impairment
-
Severe renal impairment
-
Pregnancy
-
Initiation of Janus kinase inhibitors should be avoided in patients with absolute neutropenia (less than 1000 cells/mm3) or absolute lymphopenia (less than 500 cells/mm3)
-
Active infection
-
Cautious use is recommended in patients with cardiovascular risk factors including prior history of stroke, myocardial infarction
-
Cautious use is recommended in patients with prior history of venous thromboembolism including prior history of pulmonary embolism or deep venous thrombosis
-
Abrocitinib is contraindicated in patients taking anti-platelet therapies except low-dose Aspirin (81 mg) during the first three months of treatment
Adverse effects of Janus kinase inhibitors
Janus kinase inhibitors are considered to have a similar safety profile compared with the conventional synthetic disease-modifying anti rheumatic drugs. Listed below are some of the most common and important side effects of Janus kinase inhibitors:
Increased risk of infections
While the most commonly reported adverse events include an increase in the risk of infections like upper respiratory tract infections and urinary tract infections, risks for serious infections such as tuberculosis, herpes zoster and other opportunistic infections are probably comparable to b disease-modifying anti rheumatic drugss. 37–40 Incidence for tuberculosis is similar to b disease-modifying anti rheumatic drugs which is around 0.1 to 0.22 per 100 patient years; hence, screening for latent tuberculosis should be performed prior to starting Janus kinase inhibitors. Tofacitinib was noted to have higher risk for herpes zoster infections 41–45 compared to the disease-modifying anti rheumatic drugs at a rate of 3.4 per 100 patient years. Hence, pre-vaccination against herpes zoster is recommended prior to initiation of Janus kinase inhibitors therapy whenever possible.
Other opportunistic infections like oesophageal candidiasis, cryptococcal and cytomegalovirus infections have been reported at a rate comparable to b disease-modifying anti rheumatic drugs at 0.1–0.3 per 100 patient years. Performing Janus kinase inhibitors therapy is recommended on diagnosis of these infections and initiation of infection-specific treatment. Though there are no definite guidelines on when to resume the therapy after clearing the infection, it should be evaluated on a case-to-case basis.
Gastrointestinal: Elevation in liver enzymes has been reported in patients on Janus kinase inhibitors therapy, especially if it is used in combination with other disease-modifying anti rheumatic drugs including methotrexate or TNF inhibitors. 46–58
While side effects like nausea and diarrhoea have been reported in patients with tofacitinib, the most dreadful complication reported is gastrointestinal perforation, especially in patients on NSAIDs or glucocorticoid therapy in addition to Janus kinase inhibitors. Thus, it is recommended to avoid Janus kinase inhibitors in patients with a history of diverticulitis if possible.
Haematologic: All Janus kinase inhibitors are associated with modest neutropenia and lymphopenia without significant anaemia. Statistically higher rates of thromboembolic events like deep venous thrombosis and venous thromboembolism are seen in patients treated with Janus kinase inhibitors as compared to placebo. 59
Oncologic: In a clinical trial comparing the malignancy risk in patients on tofacitinib versus TNF inhibitors, it was found that patients on tofacitinib are at a higher risk for malignancy, especially lymphoma and lung cancer. 60,61 On the other hand, further studies have not shown any potential risks for malignancy when comparing Janus kinase inhibitors with conventional disease-modifying anti rheumatic drugs like methotrexate. 43,47
Cardiovascular: Janus kinase inhibitors have been associated with dyslipidaemia, typically elevation in high-density lipoprotein, low-density lipoprotein and total cholesterol, 12 weeks after starting the treatment. It is therefore recommended to monitor lipid panel while initiating treatment with Janus kinase inhibitors.
Reproductive health: Janus kinase inhibitors are pregnancy category C with limited human studies meaning they should be used only if risks outweigh the benefits. Animal studies with filgotinib have shown impaired spermatogenesis and decreased fertility. 62 This influenced FDA’s decision of not approving filgotinib in the United States and it is currently approved only in Europe and Japan with special warnings.
Summary
Janus kinase inhibitors are exemplary novel small molecules which have been noted to be useful in various immune-mediated diseases.
-
1.
The JAK-STAT signalling pathway regulates the production of several cytokines involved in the proliferation and activation of effector T cells which play an integral role in the pathogenesis of several immune-mediated diseases.
-
2.
Given that these molecules block various signalling pathways simultaneously, they are noted to play a pivotal role in several autoimmune diseases with higher potency and better safety profile, especially with the more targeted newer generation Janus kinase inhibitors.
-
3.
Several Janus kinase inhibitors have already been approved in various immune-mediated conditions including atopic dermatitis, alopecia areata, psoriasis, psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, amongst others. There are several ongoing trials for discovering new molecules as well as their roles in other immune-mediated diseases.
Acknowledgments
This project was supported by the VA Sacramento Medical Center.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflict of interest
There is no conflict of interest.
References
- Janus kinase-signal transducers and activators of transcription cell signaling in Spondyloarthritis: rationale and evidence for JAK inhibition. Curr Opin Rheumatol. 2021;33:348-55.
- [CrossRef] [PubMed] [Google Scholar]
- A decade of JAK inhibitors: what have we learned and what may be the future? Arthritis Rheumatol. 2021;73:2166-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Janus kinase/signal transducer and activator of transcription pathways in spondyloarthritis. Curr Opin Rheumatol. 2017;29:311-16.
- [CrossRef] [PubMed] [Google Scholar]
- FR255734, a humanized, Fc-silent, anti-CD28 antibody improves psoriasis in the SCID mouse-psoriasis xenograft model. J Invest Dermatol. 2008;128:1969-76.
- [CrossRef] [PubMed] [Google Scholar]
- Studying interactions between dendritic cells and T cells in vivo. Curr Opin Immunol. 2019;58:24-30.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Polyfunctional TEM cells in psoriatic arthritis synovium skewed towards Th17 cells. Ann Rheum Dis. 2022;81:e5.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanistic rationales for targeting interleukin-17A in spondyloarthritis. Arthritis Res Ther. 2017;19:51.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IL-9 receptor: Regulatory role on FLS and pannus formation. Cytokine. 2018;111:58-62.
- [CrossRef] [PubMed] [Google Scholar]
- Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(Suppl 1):i4-i16.
- [Google Scholar]
- Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a systematic literature research. RMD Open. 2020;6:e001374.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Targeting the Janus Kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-26.
- [CrossRef] [PubMed] [Google Scholar]
- Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57:5023-38.
- [CrossRef] [PubMed] [Google Scholar]
- IL-9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine. 2016;79:45-51.
- [CrossRef] [PubMed] [Google Scholar]
- Drugs. JAK-STAT signaling as a target for inflammatory and autoimmune diseases. Current and Future Prospects. 2017;77:521-46.
- [Google Scholar]
- Blockade of the JAK/STAT pathway inhibits inflammation and bone formation in two murine models of spondyloarthritis. Arthritis Rheumatol. 2018;70(Suppl. 10)
- [Google Scholar]
- Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8:363.
- [Google Scholar]
- Inhibiting ex-vivo Th17 responses in Ankylosing Spondylitis by targeting Janus kinases. Sci Rep. 2018;8:15645.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- TYK2 inhibition reduces type 3 immunity and modifies disease progression in murine spondyloarthritis. J Clin Invest. 2020;130:1863-78.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Analysis of JAK2 and STAT3 polymorphisms in patients with ankylosing spondylitis in Chinese Han population. Clin Immunol. 2010;136:442-6.
- [CrossRef] [PubMed] [Google Scholar]
- Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Gen. 2012;90:636-47.
- [Google Scholar]
- Investigation of JAK2, STAT3 and CCR6 polymorphisms and their gene-gene interactions in inflammatory bowel disease. Int J Immunogenet. 2012;39:247-52.
- [CrossRef] [PubMed] [Google Scholar]
- JAK3, severe combined immunodeficiency, and a new class of immunosuppressive drugs. Immunol Rev. 2005;203:127-42.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory activity and neutrophil reductions mediated by the JAK1/JAK3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond). 2010;7:41.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Podoplanin expression in wound and hyperproliferative psoriatic epidermis: Regulation by TGFβ and STAT3 activating cytokines, IFNγ, IL6, and IL22. J Dermatol Sci. 2012;65:134-40.
- [CrossRef] [PubMed] [Google Scholar]
- The JAK inhibitor tofacitinib suppresses synovial JAK1STAT signalling in rheumatoid arthritis. Ann Rheum Dis. 2015;71:440-7.
- [Google Scholar]
- Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis. 2016;75:311-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Regulatory role of the JAK STAT kinase signaling system on the IL-23/IL-17 cytokine axis in psoriatic arthritis. Ann Rheum Dis. 2017;76:e36.
- [CrossRef] [PubMed] [Google Scholar]
- US Food and Drug Administration Approval: Ruxolitinib for the treatment of patients with intermediate and high risk Myelofibrosis. Clin Cancer Res. 2012;18:3212-7.
- [CrossRef] [PubMed] [Google Scholar]
- Selective JAKinibs:Prospects in inflammatory and autoimmune diseases. Bio Drugs. 2019;33:15-32.
- [Google Scholar]
- The arrival of JAK inhibitors: advancing the treatment of immune and hematologic disorders. Bio Drugs. 2013;27:431-8.
- [Google Scholar]
- Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21:183.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298.
- [CrossRef] [PubMed] [Google Scholar]
- JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019;18 https://doi.org/10.1016/j.autrev.2019.102390
- [Google Scholar]
- Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760-70.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years:integrated analysis of data from the global clinical trials. Ann Rheum Dis. 2017;76:1253-62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- FRI0123: Safety profile of Baricitinib for the treatment of Rheumatoid Arthritis up to 8.4 years: an updated integrated safety analysis. Ann Rheum Dis. 2020;79(Suppl. 1):638.
- [Google Scholar]
- THU0187: Safety profile of Upadacitinib up to 3 years of exposure in patients with Rheumatoid Arthritis. Ann Rheum Dis. 2020;79(Suppl. 1):315.
- [Google Scholar]
- Real world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1843.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Herpes zoster and tofacitinib: Clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 2017;69:1960.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21:89.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Risk for herpes zoster in Tofacitinib-treated glucocorticoids. Arthritis Care Res. 2019;71:1249-54.
- [Google Scholar]
- Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet. 2017;390:457.
- [CrossRef] [PubMed] [Google Scholar]
- Safety profile of baricitinib for the treatment of rheumatoid arthritis over a median of 3 years of treatment: an updated integrated safety analysis. Lancet Rheumatol. 2020;2:e347.
- [Google Scholar]
- Efficacy and safety of long-term bricitinib with and without methotrexate for the treatment of rheumatoid arthritis: experience with baricitinib monotherapy continuation or after switching from methotrexate monotherapy or baricitinib plus methotrexate. Arthritis Care Res (Hoboken). 2020;72:1112.
- [CrossRef] [PubMed] [Google Scholar]
- Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71:1788.
- [CrossRef] [PubMed] [Google Scholar]
- Relative efficacy and safety of tofacitinib, baricitinib, upadacitinib and filgotinib in comparison to adalimumab in patients with active rheumatoid arthritis. Z Rheumatol. 2020;79:785.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;78:1454.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: The FINCH 2 randomized clinical trial. JAMA. 2019;322:315.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3) Ann Rheum Dis. 2019;78:1320.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Changes in serum creatinine in patients with active rheumatoid arthritis treated with tofacitinib: results from clinical trials. Arthritis Res Ther. 2014;16:R158.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Peficitinib, a JAK Inhibitor, in combination with limited conventional synthetic disease-modifying antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2017;69:932.
- [CrossRef] [PubMed] [Google Scholar]
- Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. 2021;80:727.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78:1305.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021;80:848.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol. 2019;71:892-900.
- [CrossRef] [PubMed] [Google Scholar]
- FDA Drug Safety Communication. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- EMA/PRAC Recommendations for update of the product information for tofacitinib. July 5, 2021. Available at: https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-7-10-june-2021-prac-meeting_en.pdf.
- Assessment report. International non-proprietary name: filgotinib. Available at: https://www.ema.europa.eu/en/documents/assessment-report/jyseleca-epar-public-assessment-report_en.pdf






