Translate this page into:
Topical treatment of pyoderma gangrenosum: A systematic review
Corresponding author: Dr. Harry Donnelly, Department of Medicine, St Bernard’s Hospital, Harbour Views Rd, Gibraltar-GX11 1AA, Gibraltar. harry.donnelly1@nhs.net
-
Received: ,
Accepted: ,
How to cite this article: Donnelly H, Boffa MJ. Topical treatment of pyoderma gangrenosum: A systematic review. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_700_2023
Abstract
Systemic immunosuppressants are the mainstay of treatment for pyoderma gangrenosum (PG), but they generally have significant side effects which may be avoided by limiting treatment to topical therapy. This review aimed to assess the efficacy and safety of topical treatments for PG.
An extensive literature search identified nineteen suitable publications for analysis, including two open cohort studies, five case series and twelve single case reports. The quality of evidence in the publications was graded and data relating to topical PG treatment was extracted. The lack of randomised clinical trials investigating topical monotherapy for PG means that robust statistical analysis was not possible.
The greatest weight of the current evidence for topical therapy favours either corticosteroids or calcineurin inhibitors. According to our review, both these options appear well tolerated with a few side effects and may have similar efficacy in speeding up the resolution of PG ulcers. Topical therapy could be considered for use in combination with systemic treatment. There may also be a role for isolated topical monotherapy in selected patients with PG, especially those with early or mild disease and those with idiopathic PG. However further research is needed to confirm this and establish optimal treatment approaches for this condition.
Keywords
Pyoderma gangrenosum
topical treatment
corticosteroids
calcineurin inhibitors
Introduction
Pyoderma gangrenosum (PG) is a neutrophilic dermatosis characterised, in the classic ulcerative form, by painful and rapidly evolving ulceration, typically with a violaceous, undermined border. PG ulcers may reach a large size and the ulcer base may exhibit a purulent exudate, necrosis, and granulation tissue. Classic PG ulcers may be single or multiple and affect any body site, particularly the lower limbs, and characteristically heal with atrophic, cribriform scarring. Less common variants include parastomal, pustular, bullous, and superficial granulomatous PG. PG is rare with an incidence of 3 to 10 per million per year and may be associated with systemic conditions, such as inflammatory bowel disease (IBD), rheumatoid arthritis, and haematological disorders.1,2
The pathogenesis of PG is complex and incompletely understood and this has hindered the development of specific treatment for the condition. Furthermore, the available evidence base for PG management is mostly anecdotal and thus subject to publication bias. There are only a few randomised trials comparing therapeutic options for PG and no standardised protocol to guide treatment.3 A general principle is that mild disease may be treated with topical or intralesional interventions alone, whereas more severe disease usually requires either systemic treatment or combined topical and systemic therapy.4 Management of PG in an individual case will also depend on patient comorbidities, associated diseases, and the site and extent of the lesions.5 To our knowledge, there are two comprehensive literature reviews focusing on topical treatment in PG.6,7 The lack of robust evidence for particular treatments and how they should be used for varying severity of PG makes informed decisions for clinicians and patients alike challenging and a large degree of clinical discretion and expertise may be required to decide the best management in an individual patient.
The mainstay of systemic treatment for PG is via immunosuppression and the commonly used agents are corticosteroids, ciclosporin, mycophenolate mofetil, azathioprine, intravenous immunoglobulin and, increasingly, biologic therapy, primarily anti-tumour necrosis factor (TNF) agents such as infliximab, adalimumab, and etanercept. The latter may be especially useful in patients with concomitant IBD. All these systemic options have potential severe side effects which could be largely avoided by limiting the treatment to topical therapy. The primary objectives of this review were to assess the efficacy, safety, and evidence supporting the use of topical treatments for PG.
Materials and Methods
A thorough online search was conducted for English language publications using PubMed, MEDLINE, and EMBASE. The search string was pyoderma gangrenosum and local or pyoderma gangrenosum and topical. The dates included were from inception until October 2022. Publications not relating to PG or not specific to topical treatment for PG were excluded. References of the retrieved articles were searched for additional publications relevant to the review not identified in the initial search. Given the relative rarity of PG, the inclusion criteria for publications were expanded beyond randomised controlled trials and meta-analyses to also include relevant case reports and case series. Figure 1 describes the methodology used in this review.
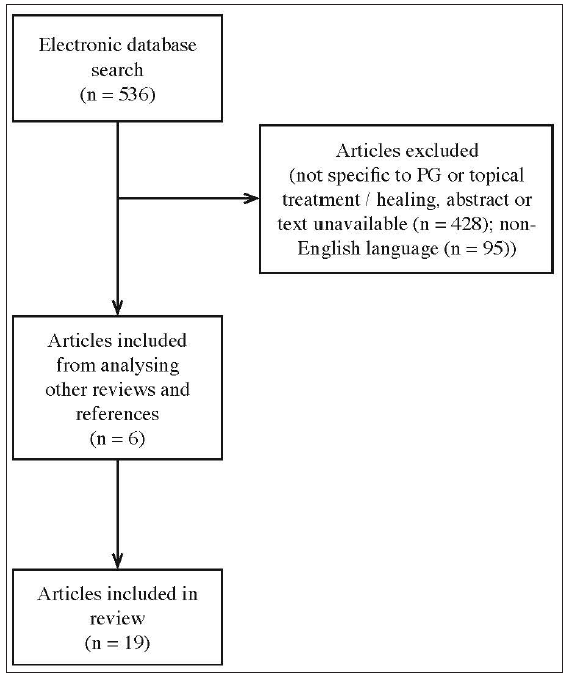
- Flowchart of review methodology.
The quality of evidence was assessed using the GRADE methodology.8 The domains involved in the GRADE assessment included risk of bias, imprecision, inconsistency, indirectness, and publication bias. The outcomes following the GRADE assessment were also included [Table 1]. Nineteen publications formed the active pool from which data was extracted and entered into a spreadsheet with the following column headings: title, year of publication, authors, type of study, demographics, biopsy confirmation of diagnosis, treatment intervention, duration of follow-up, results, and level of evidence.
| Title | Year | Authors | Type of study | Demographics | Biopsy / subtype | Interventions | Duration of follow up | Results | Level of evidence | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|
| Corticosteroids | ||||||||||
| Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study | 2016 | Thomas et al.9 | Prospective cohort study | 64 patients (44 = female), mean age = 57 | Variable, Pustular or granulomatous types excluded | Clobetasol n = 47, tacrolimus n = 10, other therapy n = 8 (other corticosteroids n = 6; fludroxycortide n = 1; lymecycline n =1) | 6 months | Clobetasol n = 20 (42.6%) healed at 6 months (median time to healing 136 days); tacrolimus n = 5 (50%) healed at 6 months (median time to healing 161 days) | 3 | Low |
| Treatment of postsurgical pyoderma gangrenosum with a high-potency topical steroid | 2010 | Hawryluk et al.15 | Case report | 1 patient, female, age 30 | ? | Topical clobetasol for 9 weeks | N/a | Gradual healing at 9 weeks | 5 | Very low |
| Topical tacrolimus in the management of peristomal pyoderma gangrenosum | 2001 | Lyon et al.10 | Cohort study | 24 patients (demographics unspecified) | Peristomal | 13 patients received clobetasol 0.05%, 11 patients tacrolimus 0.3% | N/a | 5 patients in the clobetasol group healed completely (6.5 weeks mean healing time); 7 patients in the tacrolimus group healed completely (5.1 weeks mean healing time) | 3 | Low |
| Calcineurin inhibitors | ||||||||||
| Pyoderma gangrenosum following breast reduction: treatment with topical tacrolimus and steroids | 2014 | Doren and Aya-ay17 | Case report | 1 patient, female, age 61, right breast post operatively | No | IV steroids, topical 0.1% tacrolimus given on postoperative day 13 until day 45 post-op (stopped due to high serum levels) | 8 months, healed wounds and no recurrence | Wound healing progressed rapidly, and the patient was discharged on oral prednisone taper and topical tacrolimus on postoperative day 25 | 5 | Very low |
| Successful treatment of localized pyoderma gangrenosum with topical pimecrolimus | 2012 | Cecchi et al.18 | Case report | 1 patient, female, age 56, multiple sclerosis | Abundant neutrophils in the dermis | Pimecrolimus 1% twice daily | Remained healed at 8 months | Good resolution by 21 days, Complete healing at 6 weeks | 5 | Very low |
| Topical tacrolimus for the treatment of localized, idiopathic, newly diagnosed pyoderma gangrenosum | 2009 | Marzano et al.11 | Case series | 5 patients, females, age 13-78, all idiopathic | ? / ulcerative | Tacrolimus monotherapy (dose and frequency unspecified) for 6 weeks (4-8 weeks) | 13 months, complete remission | Complete clinical remission in all patients | 4 | Low |
| FK-506 ointment: an effective adjuvant therapy to treat a dramatic case of pyoderma gangrenosum of unilateral hand | 2009 | Stefano et al.19 | Case report | 1 patient, male, age 67, hand | Bullous | 40mg prednisolone plus FK506 (tacrolimus ointment) twice daily for 3 weeks, then once daily from weeks 3 to 12 | 6 months, no recurrence | Initial improvement in 72 hours, with complete healing by week 12 | 5 | Very low |
| Successful treatment of severe pyoderma gangrenosum with pimecrolimus cream 1% | 2008 | Bellini et al.20 | Case report | 1 patient, female, age 72, ulcerative colitis | Dense and diffuse neutrophilic infiltrate | Pimecrolimus 1% twice a day. Also receiving 50mg daily prednisolone for myalgia (developed PG while taking prednisolone) | “No recurrence of lesions was observed during the 12-month follow-up period” | “After 15 days of the pimecrolimus treatment, a positive effect (reduction in pain, erythema, and ulcer size) was evident. Complete healing of ulcers of the legs and elbows was achieved after 8 and 12 weeks, respectively, despite the gradual decrease and discontinuation of oral prednisone” | 5 | Very low |
| An open-label study of topical tacrolimus ointment 0.1% under occlusion for the treatment of pyoderma gangrenosum | 2006 | Kontos et al.21 | Case series | 5 patients, 4 female 1 male, age 33-47 | Yes, unspecified | 0.05% tacrolimus ointment twice daily for 16 weeks | N/a | 2 patients healed completely by week 16, 3 patients withdrew (worsening ulcer) | 4 | Low |
| Topical tacrolimus therapy for pyoderma gangrenosum | 2005 | Chiba et al.22 | Case report | 1 patient, male, age 54 | ? | Oral prednisolone, then topical tacrolimus | N/a | Lesion regression at 2 months | 5 | Very low |
| Efficacy and systemic absorption of topical tacrolimus used in pyoderma gangrenosum | 2004 | Ghislain et al.14 | Case report | 1 patient, male, age 77, surgical site (total hip replacement) | Massive infiltration with neutrophils | Systemic ciclosporin (IV 5mg/kg/day), no improvement at 15 days - topical tacrolimus 0.1% once daily introduced. Reduced to 0.03% at day 19 (serum tacrolimus levels high) | N/a | Highly beneficial, with pain relief and ulcer regression beginning the day after tacrolimus introduction. Complete healing at 6 weeks | 5 | Very low |
| Other therapy | ||||||||||
| Topical timolol for the treatment of pyoderma gangrenosum | 2017 | Moreira et al.23 | Case report | 1 patient, female, age 46, periumbilical, collagenous colitis and ankylosing spondylitis | ? | Four drops of 0.5% timolol maleate ophthalmic solution applied on alternate days | 125 days, no recurrence | Significant improvement seen at 40 days | 5 | Very low |
| Two percent topical phenytoin sodium solution in treating pyoderma gangrenosum: a cohort study | 2010 | Fonseka et al.24 | Case series | 6 patients, 2 male and 4 female, age 25-57. 3 patients had idiopathic PG and 3 had underlying conditions | 3/6 patients - neutrophilic vascular reaction, necrosis with mononuclear cell infiltrate, fibrosing inflammation | Five of the patients were receiving systemic treatment with combinations of prednisolone, azathioprine and cyclosporin. All patients were given topical betamethasone prior to starting topical phenytoin 2% | N/a | At 4 weeks 100% improvement (as measured by ulcer size) in four patients (including the patient not receiving systemic immunomodulation, 60% improvement in one patient and 20% improvement in one patient | 4 | Low |
| Successful treatment of pyoderma gangrenosum with topical 0.5% nicotine cream | 2004 | Patel et al.25 | Case series | 2 patients, female, ages 52 and 67 | “Extensive neutrophil- rich superficial dermal infiltrate” | Patient 1: 60mg prednisolone plus 0.5% nicotine cream. Patient 2: 5mg prednisolone (longstanding) plus 0.5% nicotine cream | N/a | Patient 1: improvement at 5 days (prednisolone discontinued), complete healing at 8 weeks. Patient 2: improvement at 5 days, complete healing at 4 weeks | 5 | Very low |
| Topical platelet-derived growth factor accelerates healing of myelodysplastic syndrome-associated pyoderma gangrenosum | 2002 | Braun-Falco et al.26 | Case report | 1 patient, female, age 53, dorsum right hand, myelodysplastic syndrome | Dense neutrophilic inflammation with focal admixture of a few atypical lymphoid cells | Oral methylprednisolone 80mg daily for 3 weeks, then 40mg daily plus PDGF | Patient died of acute internal bleeding 2 weeks after wound healing | Granulation at 4 weeks, epithelialisation at 6 weeks, almost complete wound close at 9 weeks | 5 | Very low |
| Topical treatment with 1% sodium cromoglycate in pyoderma gangrenosum | 1996 | Tamir et al.27 | Case series | 5 patients, age 25-30, hospitalised | ? | 1% sodium cromoglycate. Systemic steroids added to 2 patients due to inadequate response | N/a | Initial improvement was noted in all 5 patients after 3–7 days of sodium cromoglycate treatment. Complete healing of the ulcers occurred within 5–8 weeks | 4 | Low |
| Successful treatment of pyoderma gangrenosum with topical 5-aminosalicylic acid | 1993 | Sanders and Hulsmans28 | Case report | 1 patient, female, age 29, left lower leg, Crohn’s disease | Dense dermal neutrophilic infiltrate with necrosis at the ulcer edge | 60mg prednisolone started for Crohn’s flare alongside once daily application of 10% 5-aminosalicylic acid, reduced to three times a week after initial improvement | N/a | Complete recovery in 5 weeks | 5 | Very low |
| Pyoderma gangrenosum—response to topical nitrogen mustard | 1992 | Tsele et al.29 | Case report | 1 patient, male, age 69, right ankle, IgA monoclonal gammopathy | Intense neutrophilic infiltrate | Initially treated with systemic steroids and then plasmapheresis, 20% topical nitrogen mustard started when vascular access failed | N/a | Complete healing at 3 months, and remained healed ever since | 5 | Very low |
| Treatment of pyoderma gangrenosum with benzoyl peroxide | 1977 | Nguyen and Weiner30 | Case report | 1 patient, female, age 53, right buttock | ? | Local treatment with benzoyl peroxide (20%) lotion | N/a | Clearing of the cutaneous lesion in about six weeks | 5 | Very low |
IV: intravenous, PDGF: platelet-derived growth factor,?: unspecified, N/a: not available
Results
The records included two cohort studies (n = 88), five case series (n = 23), and twelve individual case reports (n = 12). The total number of participants included in the literature review was thus 123. The gender and age range were not specified in every publication, but of the data available, the demographics included: 76 women and 27 men with a mean age of 52.1 years. Biopsy results were mentioned in seven (58%) of the patients in the case reports and in ten (43%) of the total patients in the case series.
In the larger cohort study, 41 patients (64%) were found to have an underlying condition associated with PG.9 In the second cohort study, all patients were classified as having peristomal PG which is typically associated with IBD, but the precise conditions were not delineated.10 Of the remaining reports included in the review, an underlying condition was described in ten participants (29%), eight cases were termed idiopathic (23%) and the remainder were unspecified. Therefore, of all participants included in the review, 51–75 (41–61%) had an identified underlying condition. A summary of all the included publications is shown in Table 1.9-11,14-15,17-30
Discussion
There are no randomised controlled trials related to topical treatments for PG. This is not surprising as the severity and morbidity of the condition would make a placebo arm in any such trial difficult to rationalise. Our review involved twelve case reports, five case series, and two small cohort studies, and in most of the cases topical treatment was with either corticosteroids or calcineurin inhibitors. The most frequently used agent in each class was clobetasol propionate and tacrolimus, respectively. Table 1 shows that the overall quality of evidence was low to very low when assessed via GRADE criteria. This is not unexpected, given the low incidence of PG and therefore a relative reliance on case reports and expert opinion pieces in the literature. The lack of high-quality randomised data means that in-depth statistical analysis is not possible. Nevertheless, broad comparisons can still be made about the efficacy and safety of the more frequently observed topical therapies.
Table 2 shows that the percentage of patients achieving complete healing is comparable between the corticosteroid group (42.6%) and the calcineurin inhibitor group (67.5%). However, a theme common to the included publications is that the parameters of complete healing are not well defined. Variously used metrics in the literature included ulcer size, severity, and subjective clinician and patient satisfaction but precise measurements of the ulcer size and shape were rarely reported. The larger cohort study included in the review presented measurements in the form of median ulcer area and global disease severity presented both via clinician-assessed reduction in erythema and patient quality of life scores (Dermatology Life Quality Index, DLQI, and the EuroQol index, EQ-5D-5L).9 Standardised reporting such as this is likely to yield more meaningful results and a similar framework should be considered in future studies.
| Corticosteroids (n = 61) | Calcineurin inhibitors (n = 37) | |
|---|---|---|
| Agents used | Clobetasol propionate, n = 61 | Tacrolimus, n = 35; pimecrolimus, n = 2 |
| Number of patients with complete healing | n = 26 (42.6%) | n = 25 (67.5%) |
| Mean healing time | 118.4 days (SD 37.1) | 79.1 days (SD 56.0) |
SD: Standard deviation
The mean healing time in the calcineurin inhibitor group (79.1 days) was less than in the corticosteroid group (118.4 days).
The results from Thomas et al. showed a picture similar to this review, in terms of treatment success for topical clobetasol (42.6%) and tacrolimus (50%).9 However, the design of the study did not allow for direct statistical comparisons between the two topical treatments. Treatment arms were not randomised and there were no strictly stipulated inclusion and exclusion criteria. The study was also hindered by comparatively small sample sizes, but a finding that slightly less than half of the participants (43.8%) progressed to ulcer healing with topical therapy alone is encouraging. The authors also suggested that the size of the ulcer at the time of treatment onset is an important predictor of overall healing time. This is a claim unique to this study and one which warrants further investigation.
In the other cohort study, systemic treatments were introduced when topical treatment failed or the initial presentation of PG was considered too severe for topical treatment alone.10 The systemic treatments included prednisolone 0.5–1mg/kg and ciclosporin 3.5mg/kg, but it was not reported how many participants received either treatment. The majority of synthesised data for both the corticosteroid and calcineurin inhibitor groups analysed in this review is taken from the two aforementioned cohort studies.
The case series from Marzano et al. reported tacrolimus monotherapy in five patients with newly diagnosed PG.11 A weakness of the series was that although the cases are listed as having ulcerative PG, there was no clarification of the biopsy status. The dose of tacrolimus was also not mentioned. Two points worth noting from the series are that PG was considered to be idiopathic and ulceration was less than 1 month old when topical therapy was started, in all patients. The inference is that idiopathic and early PG may be more amenable to local therapy than systemic and longstanding PG. The mechanism by which this could be the case, aside from the fact that early PG has had less time to evolve and become more severe, is unclear and warrants further study.
The relative efficacy of topical treatments for the different subtypes of PG is not easy to assess from the data available across the review as subtypes were rarely identified in the reports. Similarly, the presence or absence of underlying conditions associated with PG was not uniformly mentioned and so the relative efficacy of topical treatments in treating co-morbid versus idiopathic PG cannot be measured. However, in the patients described as having idiopathic PG (n = 8), there was improvement seen in all cases on initiation of topical therapy (tacrolimus n = 5, phenytoin n = 3). The delineation between co-morbid and idiopathic PG may thus be useful in predicting which patients might fare better with topical therapy. On the other hand, a confounding factor is that patients with underlying conditions who develop PG may already be taking systemic immunosuppression and so the effect of adding topical therapy may be hard to isolate. A trend in the literature towards using topical treatment for peristomal and less extensive disease may suggest increased utility in these subsets of PG patients.9,10,11
In the publications utilising topical corticosteroids, no specific steroid adverse effects were mentioned. One explanation could be that local corticosteroid side effects (e.g. atrophy, purpura, and ulceration) may be less evident in skin already ulcerated due to PG. Topical corticosteroids have been shown to be well tolerated and safe overall, but as with topical calcineurin inhibitors, systemic absorption of corticosteroids is increased in diseased skin.12 As such, clinicians should be alert to potential systemic effects when treating PG with prolonged courses of highly potent steroids, particularly if applied under occlusion which could increase absorption.
The primary indication for topical tacrolimus is atopic dermatitis in which the commonest adverse effect is transient local irritation and burning.13 In our review, a transient burning sensation was similarly the only tacrolimus side effect reported. The most serious consequence of tacrolimus toxicity is acute kidney injury; however, systemic side effects are known to be very rare when topical tacrolimus is applied to intact skin, as systemic absorption is low.14 In one patient in our review, topical tacrolimus 0.1% had to be reduced to 0.03% after 7 days as tacrolimus level was high and creatinine levels increased.14 However, as the patient was also on ciclosporin, it cannot be said whether it was the tacrolimus, ciclosporin, or a combination of both that precipitated nephrotoxicity and, reassuringly, creatinine returned to baseline levels after a dose reduction in both treatments. Nevertheless, it would appear prudent to advise monitoring of tacrolimus and creatinine levels during prolonged treatment in patients with PG.
There were no major safety concerns with any of the reported topical treatments and in no case was treatment withdrawn due to adverse events. Topical corticosteroids and calcineurin inhibitors can be considered to be a generally safe and well-tolerated component in the clinician’s arsenal for the treatment of PG.
Limitations
The main limitation of our review is the limited availability of high-quality evidence in the literature. In fact, all the included publications were deemed to be of either very low or low quality when assessed by the GRADE criteria and the only two available cohort studies were relatively small, open-label, without randomisation, and relatively old. The review is further limited by the fact that most of the studies have not described topical therapy in isolation. The larger cohort study aimed to compare topical monotherapy with either clobetasol or tacrolimus; however, even amongst the topical clobetasol group (n = 47), five patients were receiving systemic immunosuppression for other conditions (azathioprine (n = 2), tetracyclines (n = 2) and anti-TNF (n = 1)).
Furthermore, around half of the cases (n = 16) in the calcineurin inhibitor group (n = 37) came from case reports or case series. As such, there is a large degree of publication bias present in that group compared to the topical corticosteroid group, where only one case report was included and the other patients came from the cohort studies. The difference in data sources for the two treatment groups could be another source of bias in our review. Female preponderance in our review is in line with published data and thus unlikely to represent a significant limitation.
Conclusion
The objectives of our review were to evaluate the efficacy and safety of topical treatments used either alone or in combination with systemic therapy for PG. Currently, topical corticosteroids are the most commonly used topical agent. This review suggests that topical calcineurin inhibitors, particularly tacrolimus, may represent an alternative to topical corticosteroids. The absence of any randomised controlled trials and the fact that topical treatment was rarely used as a monotherapy in the available publications means that no definite answer can be drawn about the efficacy of monotherapy versus combined treatment. PG is typically rapidly progressive and has significant morbidity, thus there is an imperative to treat it as quickly and effectively as the current evidence base allows. The available evidence suggests that systemic treatments are most effective. As such, a randomised controlled trial comparing topical monotherapy with systemic treatments would be challenging to design, both ethically and logistically.
An interesting point raised in our review, albeit one highlighted by a small case series, is that topical treatment could present an appealing first-line option in patients with newly diagnosed PG that is not associated with systemic disease. Current practice tends towards swift treatment with systemic corticosteroids in such patients, but further investigation into the efficacy of topical treatment for early, idiopathic, and mild PG could be logical based on the findings of this review. With regard to the more frequently seen types of PG associated with systemic disease and recurrent PG, a prospective trial comparing topical corticosteroids and calcineurin inhibitors, perhaps with a third arm receiving standardised systemic treatment alone could also be useful. Our review highlights the need for standardised measures of PG severity prior to treatment initiation, alongside reproducible metrics of healing (e.g. pain, perilesional erythema, and stabilisation of ulcer size) and patient satisfaction, to help guide future management decisions for this difficult-to-treat and often severe condition.
Despite the limitations of our review, it would appear reasonable to present some broad highlights from the evidence analysed here regarding the topical treatment of PG:
-
The most widely used topical treatments for PG are corticosteroids and calcineurin inhibitors. The available evidence suggests that they are likely to have similar efficacy and are generally well tolerated.
-
In patients with idiopathic PG and small, early-onset lesions, there may be a greater role for topical monotherapy. For these patients, early intervention with topical treatment may present a preferable treatment option to prolonged therapy with systemic immunosuppression.
Acknowledgements
There are no acknowledgements or financial conflicts of interest to disclose for either author.
This monograph is based on a professional project submitted to the University of South Wales, UK, in part fulfillment of the requirements for the degree of MSc in Dermatology in Clinical Practice.
Declaration of patient consent
Patient’s consent not required as patients’ identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Clinical management of pyoderma gangrenosum. J Am Acad Dermatol. 2002;3:149-58.
- [CrossRef] [PubMed] [Google Scholar]
- Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment options for pyoderma gangrenosum. J Dtsch Dermatol Ges. 2017;15:34-40.
- [CrossRef] [PubMed] [Google Scholar]
- Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges. 2015;13:317-24.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- [CrossRef] [PubMed] [Google Scholar]
- A comprehensive review of local pharmacologic therapy for pyoderma gangrenosum. Wounds. 2019;31:151-7.
- [PubMed] [Google Scholar]
- Topical treatment of pyoderma gangraenosum. Dermatology. 2002;205:221-3.
- [CrossRef] [PubMed] [Google Scholar]
- GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-6.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study. J Am Acad Dermatol. 2016;75:940-9.
- [CrossRef] [PubMed] [Google Scholar]
- Topical tacrolimus in the management of peristomal pyoderma gangrenosum. J Dermatolog Treat. 2001;12:13-7.
- [CrossRef] [PubMed] [Google Scholar]
- Topical tacrolimus for the treatment of localized, idiopathic, newly diagnosed pyoderma gangrenosum. J Dermatolog Treat. 2010;21:140-3.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic side-effects of topical corticosteroids. Indian J Dermatol. 2014;59:460.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Tacrolimus: a review of its use for the management of dermatoses. J Eur Acad Dermatol Venereol. 2002;16:100-14.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and systemic absorption of topical tacrolimus used in pyoderma gangrenosum. Br J Dermatol. 2004;150:1052-3.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of postsurgical pyoderma gangrenosum with a high-potency topical steroid. Ear Nose Throat J. 2010;89
- [PubMed] [Google Scholar]
- Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-50.
- [CrossRef] [PubMed] [Google Scholar]
- Pyoderma gangrenosum following breast reduction: treatment with topical tacrolimus and steroids. Aesthet Surgery J. 2014;34:394-9.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of localized pyoderma gangrenosum with topical pimecrolimus. J Cutan Med Surg. 2012;16:295-7.
- [CrossRef] [PubMed] [Google Scholar]
- FK-506 ointment: an effective adjuvant therapy to treat a dramatic case of pyoderma gangrenosum of unilateral hand. Chin J Traumatol. 2009;12:181-3.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of severe pyoderma gangrenosum with pimecrolimus cream 1%. J Eur Acad Dermatol Venereol. 2008;22:113-5.
- [CrossRef] [PubMed] [Google Scholar]
- An open‐label study of topical tacrolimus ointment 0.1% under occlusion for the treatment of pyoderma gangrenosum. Int J Dermatol. 2006;45:1383-5.
- [CrossRef] [PubMed] [Google Scholar]
- Topical tacrolimus therapy for pyoderma gangrenosum. J Dermatol. 2005;32:199-203.
- [CrossRef] [PubMed] [Google Scholar]
- Topical timolol for the treatment of pyoderma gangrenosum. BMJ Case Rep. 2017;2017:bcr2016218589.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Two percent topical phenytoin sodium solution in treating pyoderma gangrenosum: a cohort study. Int Wound J. 2010;7:519-23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Successful treatment of pyoderma gangrenosum with topical 0.5% nicotine cream. J Dermatolog Treat. 2004;15:122-5.
- [CrossRef] [PubMed] [Google Scholar]
- Topical platelet‐derived growth factor accelerates healing of myelodysplastic syndrome‐associated pyoderma gangrenosum. Br J Dermatol. 2002;147:829-31.
- [CrossRef] [PubMed] [Google Scholar]
- Topical treatment with 1% sodium cromoglycate in pyoderma gangrenosum. Dermatology. 1996;192:252-4.
- [CrossRef] [PubMed] [Google Scholar]
- Successful treatment of pyoderma gangrenosum with topical 5-aminosalicylic acid. Cutis. 1993;51:262-4.
- [PubMed] [Google Scholar]
- Pyoderma gangrenosum—response to topical nitrogen mustard. Clin Exp Dermatol. 1992;17:437-40.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of pyoderma gangrenosum with benzoyl peroxide. Cutis. 1977;19:842-4.
- [PubMed] [Google Scholar]






