Translate this page into:
A novel method of assessing intraoperative surgical margins in patients with dermatofibrosarcoma protuberans: A specimen radiography system
Corresponding author: Dr. Jiaqi Liu, Department of Plastic Surgery, Zhongshan Hospital, Fudan University, Shanghai, China, liu.jiaqi@zs-hospital.sh.cn
-
Received: ,
Accepted: ,
How to cite this article: Wang Q, Qian L, Qi F, Liu J. A novel method of assessing intraoperative surgical margins in patients with dermatofibrosarcoma protuberans: A specimen radiography system. Indian J Dermatol Venereol Leprol. 2024;90:566. doi: 10.25259/IJDVL_959_2021
Abstract
Background
Dermatofibrosarcoma protuberans (DFSP) is one of the most challenging cutaneous cancers in surgical clinic practice. Excision with negative margins is essential for effective disease control. However, wide surgical margins and maximal tissue conservation are mutually exclusive. Mohs micrographic surgery conserves tissue but is time-consuming. Thus, we developed a novel specimen radiography system that can be used intraoperatively.
Aims
To introduce a specimen radiography system for evaluating intraoperative surgical margins in patients with dermatofibrosarcoma protuberans.
Methods
Since September 2017, we have treated seven biopsy-proven cases of local DFSPs via local excision with surgical margins of 2–4 cm. During operations, the operative specimens were screened using the specimen radiography system. All surgical specimens were pathologically examined intraoperatively.
Results
Five patients were men and two were women, of median age 36 years. The mean radiographic screening time was 9.7 ± 2.3 min. Radiographically negative margins were confirmed intraoperatively. The minimal margin width ranged from 5.0 to 35.4 mm (mean width 16.9 ± 10.4 mm). The intraoperatively negative radiographic margins were consistent with those revealed by postoperative pathology. The minimal pathological margin width ranged from 4.0 to 34.5 mm (mean 16.6 ± 10.1 mm) and was not significantly different from the intraoperative data.
Limitations
The sample size was small and positive or negative predictive values were not calculated.
Conclusions
We introduce a novel method of intraoperative surgical margin assessment for DFSP patients. It may find broad clinical and research applications during oncoplastic surgery.
Keywords
Plastic surgery
oncology
skin neoplasms
dermatofibrosarcoma protuberans
radiography
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare mesenchymal tumour of intermediate-grade malignancy. The tumour is characterised by slow infiltrative growth and a high rate of local recurrence.1 Effective surgical treatment features wide local excision (WLE) with surgical margins of 1 to 2 cm and requires negative pathological margins.2 Even after optimal preoperative evaluation, it can be difficult to determine the surgical margins required to achieve pathological negativity. The National Comprehensive Cancer Network (NCCN) and European multidisciplinary experts recommend a margin of 2–4 cm, thus up to the deep fascia or pericranium and, if clinically feasible, with confirmed pathological negativity.3 In practice, however, it is almost inevitable that such incisions will be associated with serious facial and/or functional defects, particularly in areas where the anatomy is challenging, such as the head and neck.4
Given the propensity of DFSP to recur, Mohs micrographic surgery (MMS), a technique that ensures complete margin assessment with tissue preservation, has been suggested as a treatment for DFSP.4,5 However, it is a very elaborate approach, requiring a great deal of training and a special ancillary team which limits its applications. It is also very labour-intensive, requiring the preparation and review of multiple histological tumour sections while the patient waits. For many patients, a phased surgical procedure (‘a slow Mohs’) is done which requires many hours.5 MMS is both costly and inconvenient.6,7 An ideal method should save cost and be rapid. Recently, new imaging techniques such as ex vivo confocal microscopy are developing as a fast alternative to MMS.8 Here, we introduce an intraoperative specimen radiography system that enhances the surgical management of DFSP. Although specimen radiography is established for breast tissue imaging, its use for intraoperative DFSP margin analysis is novel in the field of oncoplastic surgery.
Patients and Methods
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Zhongshan Hospital, Fudan University. All operations and procedures were performed in accordance with the relevant tenets of the Declaration of Helsinki. All patients received a detailed description of the study and gave a written informed consent.
Surgical DFSP specimens
Commencing in September 2017, we enrolled seven patients. All were operated upon under general anaesthesia. Specimens were radiographically screened in the operation room immediately after surgical excision.
Screening of surgical specimens
We used the Trident intraoperative specimen radiography system (Hologic, Marlborough, MA, USA) [Figure 1]. The lighted specimen X-ray chamber is covered by lead, thus the device can be placed in the operating room without additional radiation protection.

- View of the specimen radiography system (HOLOGIC, Marlborough, MA, USA). This mobile digital specimen radiography unit is composed of a display device, computer and screening box. The system can be easily moved into the operation room.
Immediately after excision, the specimen was placed in a specimen bag [Figure 2] and imaged at 1× magnification with automatic exposure control. The entire specimen was screened. Multiple images were acquired if needed, although no specific operational guidelines on orientation or the number of projections were mandated. More details about this device can be seen on the website: https://www.hologic.com/hologic-products/breast-health-solutions/trident-specimen-radiography-system
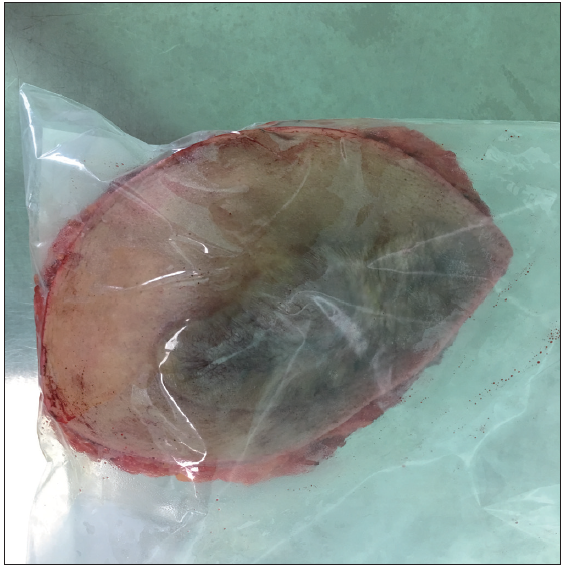
- Immediately after surgical excision, the specimen was conceptualised into a plastic bag. This plastic bag prevents specimens from contaminating the screen box without affecting image quality.
Histopathological examination
All surgical specimens were examined by pathologists who measured the minimal widths of the margins. The pathology reports were reviewed in terms of the final histopathological findings.
Margin analyses
The radiographic margins of the primary specimens were compared to the histopathological results.
Data analyses
The paired Student’s t-test was used to determine whether the minimal widths determined by our novel radiography system and conventional pathology significantly differed. A p-value < 0.05 was taken as statistically significant. All data analyses were performed using SPSS software (ver. 22.0, IBM Institute, CA, USA).
Results
Patients
Five patients were men, two were women and the median age was 36 years (range 22 to–62 years; mean age 39.9±14.7 years). Preoperatively, all patients were pathologically diagnosed with DFSP via biopsy, as shown in Table 1.
| Patient No. | Sex | Body mass index | Surgical site | Age | Operation time (min) | Detecting time (min) | Trident (mm) | Pathology (mm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Men | 25 | Trunk | 28 | 95 | 11 | 10.4 | 10 |
| 2 | Women | 21 | Left upper extremity | 32 | 85 | 9 | 12.5 | 13 |
| 3 | Women | 31 | Trunk | 56 | 88 | 12 | 17 | 18 |
| 4 | Men | 21 | Trunk | 43 | 102 | 8 | 25.7 | 24.2 |
| 5 | Men | 23 | Left upper extremity | 22 | 92 | 8 | 5 | 4 |
| 6 | Men | 29 | Trunk | 62 | 80 | 7 | 12.2 | 12.5 |
| 7 | Men | 22 | Trunk | 36 | 82 | 13 | 35.4 | 34.5 |
Surgical approaches
All patients underwent local excision with surgical margins of 2-4 cm as recommended by the NCCN and European multi-disciplinary experts. Primary closure was performed for all patients. All operations were uneventful with no major complications. Postoperative management was uneventful.
Radiology findings
The radiographic image demonstrates well-defined margins between the specimen and the surrounding normal tissues. The edges appear clear and distinct, indicating a potentially clean and complete excision during the surgical procedure. The boundaries of the DFSP specimen show no evidence of infiltration or irregularity, suggesting a high-quality surgical resection.
It is worth noting that this assessment is based on the usage of the intraoperative specimen radiography system for evaluation. In clinical practice, the interpretation of the radiographic findings would be performed by a qualified radiologist or medical professional with expertise in analysing such images. In addition, the final diagnosis and treatment decisions for DFSP should rely on a comprehensive assessment that combines radiological, pathological and clinical information.
Margin analyses
The mean duration of specimen radiography was 9.7 ± 2.3 min (range 7–13 min). Intraoperatively, negative margins were radiographically observed [Figure 3]. The minimal radiologic lateral margin width [Figure 4] ranged from 5.0 to 35.4 mm (mean 16.9 ± 10.4 mm). The negative margins were postoperatively confirmed. The minimal pathologic lateral margin width ranged from 4.0 to 34.5 mm (mean 16.6 ± 10.1 mm). No positive margins were found both with radiography and pathology.
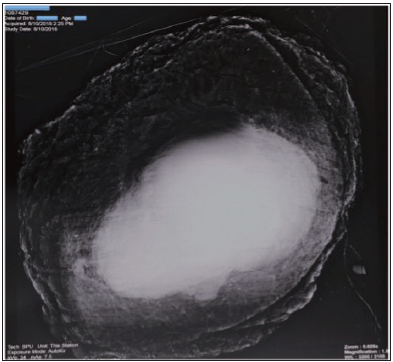
- The entire specimen of waist from a man was imaged with the system in magnification 1.0x with exposure mode of ‘AutoKy’.
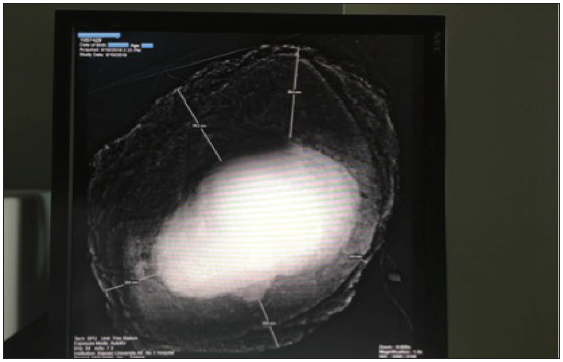
- The width of lateral margin can be gauged by scale in the specimen of waist from a man by the radiography system. The minimal lateral width can be observed and scaled.
Statistics
The intraoperatively negative radiographic margins were consistent with those seen in postoperative pathological analyses. The Student t-test found no significant differences between the two sets of data (p = 0.4388, SD of differences = 0.9118 and 95% confidence interval = −1.129 to 0.5576).
There were some limitations to our study. It was not randomised and because the sample size was small, we could not calculate positive or negative predictive values. A multi-institutional study with a larger number of patients is required. We believe that several radiologists should collaborate in a future study.
Discussion
DFSP is a rare, superficial soft tissue sarcoma. Its rarity precludes large prospective studies and thus the optimal specimen margin for the attainment of local control remains unclear. Extensive, abnormal local invasion is associated with a high recurrence rate.9 Resection margin width is strongly correlated with local DFSP control; recurrence rates are higher when margins are ‘conservative’.10,11 Surgical excision with meticulous evaluation of all margins is the mainstay of DFSP treatment and is essential to ensure a long-time cure and low morbidity.
Generally, complete DFSP surgical resection is accomplished via either MMS or a standard WLE. The choice should be dictated by the need to completely excise the carcinoma with negative margins (tantamount to a cure); to preserve function, optimise cosmesis, minimise morbidity; and to minimize cost and inconvenience to the patient and the healthcare system. Both WLE and MMS allow attainment of the first goal, but attainment of the second and third goals is more difficult.12,13 These goals pose major challenges to oncoplastic surgeons; we were thus encouraged to test a novel method and explore a new field.
Specimen radiographic devices allow rapid and effective intraoperative assessment of excised tissue. Faxitron was the first to introduce such a device in 1998. Since that time, several other systems have become available, including BioVision (Faxitron Bioptics LLC, IL, USA) and Trident. There is no need to transport specimens from the operating room to the pathology department, thereby streamlining the workflow.14 To the best of our knowledge, this is the first application of a specimen radiographic system for intraoperative margin assessments of DFSPs.
System operation is simple and brief. The differences between margins identified via intraoperative radiography and pathology was non-significant. Specimen radiography sensitively assesses surgical margins intraoperatively.
There were some limitations to our study. It was not randomised and because the sample size was small, we could not calculate positive or negative predictive values. A multi-institutional study with a larger number of patients is required. We believe that several radiologists should collaborate in a future study.
Conclusion
Intraoperative specimen radiography systems were introduced in the 1990s and are commonly employed during breast surgeries. However, no report has yet described the use of such a system during oncoplastic surgery. Here, we present our system and explore its utility.
Our method of intraoperative margin assessment during DFSP surgery is promising as the operative time is much shorter than that of the Mohs procedure. We believe that specimen radiography can be used to assess margins in patients with cutaneous soft tissue cancers such as DFSPs, basal cell carcinomas and squamous cell carcinomas. As the method expands into new areas within oncoplastic surgery, more reports with longer follow-up will, we expect, support this suggestion.
Acknowledgement
Qiang Wang performed all the experiments and prepared the manuscript. Leqi Qian and Fazhi Qi helped analyse the data. Jiaqi Liu conceptualised, planned this study.
Ethical approval
This study was approved by the Institutional Ethics Committee of Zhongshan Hospital, Fudan University. All operations and procedures were performed in accordance with the relevant tenets of the Declaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Support for the present study was funded by the National Natural Science Foundation of China (No. 81671915 and No. 81802724).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Dermatofibrosarcoma protuberans: Surgical management of a challenging mesenchymal tumour. World J Surg Oncol. 2019;17:90.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- S1 guidelines for dermatofibrosarcoma protuberans (DFSP) - update 2018. J Ger Soc Dermatol. 2019;17:663-8.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and treatment of dermatofibrosarcoma protuberans. Eur J Cancer. 2015;51:2604-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Factors, Treatment, and Survival in Dermatofibrosarcoma Protuberans. JAMA dermatology. 2016;152:1365-71.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: Systematic review. Arch dermatol. 2012;148:1055-63.
- [CrossRef] [PubMed] [Google Scholar]
- Mohs micrographic surgery for dermatofibrosarcoma protuberans: Comparison of frozen and paraffin techniques. J Eur Acad Dermatol Venereol. 2018;32:2171-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dermatofibrosarcoma protuberans: Wide local excision versus mohs micrographic surgery. Surg Oncol Clin N Am. 2016;25:827-39.
- [CrossRef] [PubMed] [Google Scholar]
- Current update on the molecular biology of cutaneous sarcoma: Dermatofibrosarcoma protuberans. Curr Treat Options Oncol. 2019;20:29.
- [CrossRef] [PubMed] [Google Scholar]
- Ex vivo confocal microscopy for dermatofibrosarcoma protuberans. Skin Res Technol. 2019;25:589-91.
- [CrossRef] [PubMed] [Google Scholar]
- Current treatment options for dermatofibrosarcoma protuberans. Expert Rev Anticancer Ther. 2015;15:901-9.
- [CrossRef] [PubMed] [Google Scholar]
- Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. 2009;135:653-65.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: The mayo clinic experience. Dermatol Surg. 2017;43:98-106.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of a mobile digital specimen radiography system for intraoperative specimen verification. AJR Am J Roentgenol. 2014;203:457-62.
- [CrossRef] [PubMed] [Google Scholar]







