Translate this page into:
Superior mesenteric artery syndrome – An unusual presentation associated with diffuse cutaneous systemic sclerosis in two cases
Corresponding author: Dr. Vishal Thakur, Department of Dermatology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. drvishal87igmc@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gowda SK, Garg S, Khajjayam A, Sahu SK, Kaur M, Patra S, et al. Superior mesenteric artery syndrome – An unusual presentation associated with diffuse cutaneous systemic sclerosis in two cases. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_315_2024
Dear Editor,
Systemic sclerosis (SSc) is a multisystem connective tissue disorder that presents with varied gastrointestinal symptoms. Gastrointestinal system can be affected from end to end with involvement ranging from decreased mouth opening, esophageal dysmotility, gastroesophageal reflux disease (GERD), reduced gastric emptying, gastric antral vascular ectasia to superior mesenteric artery syndrome (SMAS), intestinal pseudo-obstruction, small intestinal bacterial overgrowth, and anorectal dysfunction.1
Our index case is a 22-year-old woman, a case of diffuse cutaneous SSc who presented with epigastric pain, dysphagia, delayed post-prandial vomiting, and significant weight loss of about 15 kg over 6 months. Detailed history revealed Raynaud’s phenomenon for 5 years and skin tightness that started over the fingers and face and gradually progressed proximally over the forearm and trunk over 3 years. This was preceded by non-pitting oedema of bilateral fingers and forearms and salt and pepper pigmentary changes in a few areas for 9 months. There was no history of photosensitivity, oral ulcers, grittiness in eyes, or muscular weakness. On examination, the patient was pale and poorly built (weight: 30 kg). The facial skin was taut and shiny with decreased wrinkling [Figure 1]. Salt and pepper pigmentation was seen over the bilateral dorsum of hands, neck, and chest. Binding down of skin extending proximal to metacarpophalangeal joints and fixed flexion deformity of metacarpophalangeal and interphalangeal joints were observed. Abdominal examination revealed a scaphoid-shaped abdomen and absent bowel sounds. Nailfold capillaroscopy demonstrated frequent giant capillaries and mild ramified capillaries with mild disorganisation of the capillary architecture with capillary dropout. Contrast-enhanced computed tomography (CECT) of the abdomen depicted a significantly reduced aorto-mesenteric distance of 4 mm and angle of 11 degrees with distended stomach and proximal duodenal segments along with smooth tapering at the level of the D3 segment of duodenum suggesting the diagnosis of superior mesenteric artery syndrome [Figures 2 and 3]. After the failure of conservative treatment, antecolic loop gastrojejunostomy with feeding jejunostomy [Figure 4] was done. Mycophenolate mofetil was given for interstitial lung disease, and cutaneous binding down, nifedipine was given for Raynaud’s phenomenon.
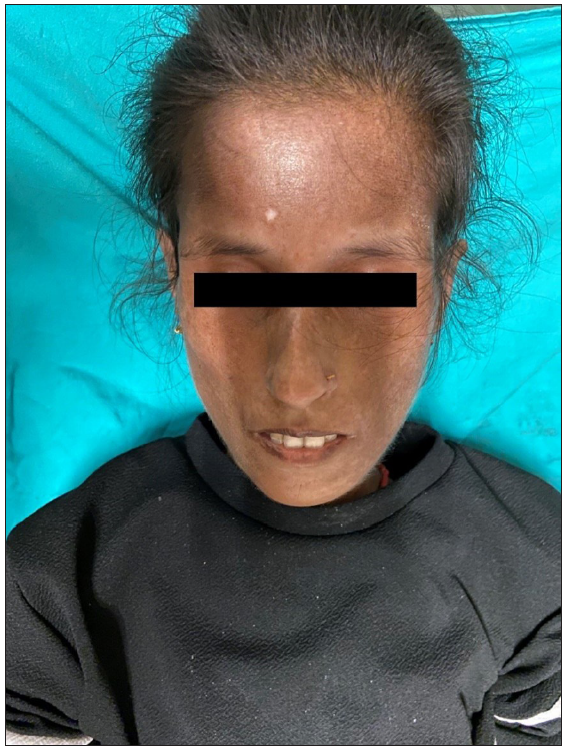
- Taut, shiny skin, decreased wrinkling of the face with salt and pepper pigmentation over the forehead.
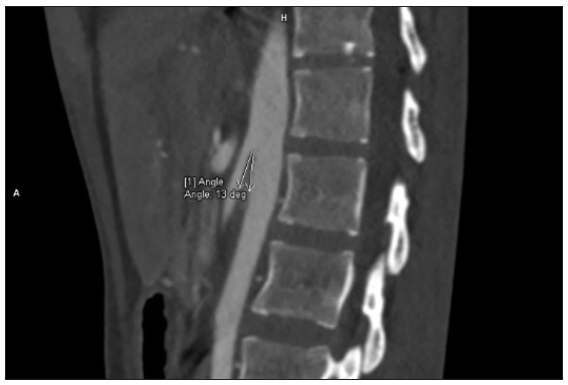
- Contrast-enhanced computed tomography of the abdomen revealed an aorto-mesentric distance of 4 mm and aorto-mesentric angle of 11 degrees suggestive of superior mesenteric artery syndrome.
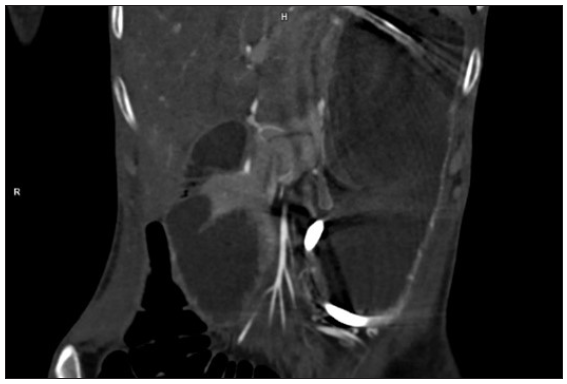
- Contrast-enhanced computed tomography of the abdomen depicts distended stomach and proximal duodenal segments along with smooth tapering at the level of the D3 segment of duodenum.
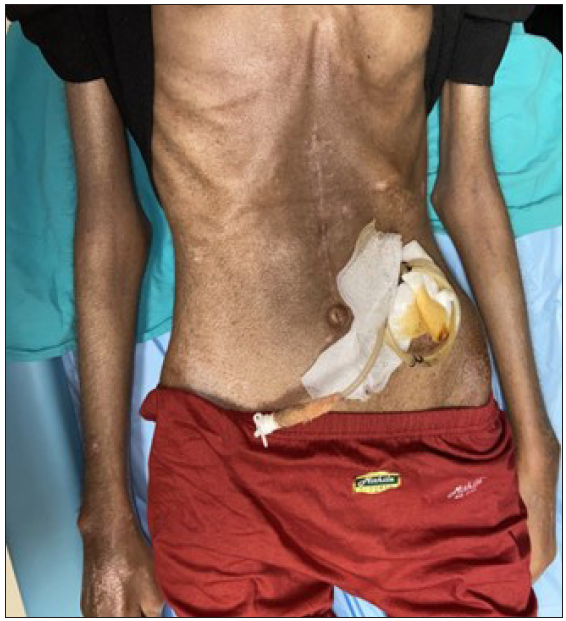
- Abdominal examination revealed scaphoid abdomen with feeding jejunostomy.
Our second case, a 52-year-old woman presented with similar cutaneous complaints for 1 year. She also gave a history of recurrent abdominal pain, bloating, and vomiting for 1 month. Examination revealed binding down of skin extending below the elbow and knee. The abdomen was distended with diffuse tenderness and rigidity with dull percussion and reduced abdominal sounds on auscultation. CECT of the abdomen confirmed the diagnosis of SMAS. Antinuclear antibody (ANA) testing showed a fine speckled pattern (2+) and anti-topoisomerase I antibodies were positive (2+). She was managed conservatively with total parenteral nutrition for 1 month. Subsequently, she was started on methotrexate and nifedipine.
SMAS is a rare vascular constriction disorder due to an anomalous course of the superior mesenteric artery (SMA) arising with acute angulation (normal >22o) from the abdominal aorta. This reduced angle results in compression of the third part of the duodenum, giving rise to obstructive symptoms. In normal circumstances, the fat and lymphatic tissues surrounding SMA give a cushion effect and protect the duodenum from being compressed.2 In SSc, loss of this cushion effect due to gastrointestinal involvement and resultant severe weight loss may result in SMAS.3 SSc is a multisystem disease where gastrointestinal involvement is seen more often in the diffuse type. The pathophysiology for gastrointestinal dysfunction includes smooth muscle infiltration by immune cells, smooth muscle fibrosis, labile vascular tone of the submucosal arterioles and venules, and dysfunction of the enteric nervous system.4 Reduced mouth opening and esophageal dysmotility leads to severe weight loss and loss of retro-peritoneal fat which is responsible for the loss of cushion effect around SMA, thereby resulting in SMAS.5
SMAS has been described in association with systemic lupus erythematosus (SLE), rheumatoid arthritis, and juvenile dermatomyositis (JDM), however, association of SMAS with SSc is only rarely reported. In SLE, gastrointestinal vasculitis resulting in severe abdominal pain, reduced food intake and weight loss has been described to cause SMAS.6 In JDM, pharyngeal muscle weakness leading to reduced food intake and weight loss has been reported to result in SMAS.7
SMAS is a rare but reversible gastrointestinal manifestation of systemic sclerosis and can be fatal if not intervened early. Hence, early diagnosis and definitive treatment either in the form of enteral or parenteral nutrition is likely to improve the nutritional status and restore the retroperitoneal fat pad. Early recognition and prompt intervention can help in decreasing the morbidity and mortality related to this condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of Interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Gastrointestinal manifestations of systemic sclerosis. J Scleroderma Relat Disord. 2016;1:247-56.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A case report of superior mesenteric artery syndrome. J Surg Case Rep. 2022;2022:rjab630.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gastrointestinal symptom severity and progression in systemic sclerosis. Rheumatology (Oxford). 2022;61:4024-34.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Intestinal obstruction and pseudo-obstruction in patients with systemic sclerosis. Acta Gastroenterol Latinoam. 2013;43:227-30.
- [PubMed] [Google Scholar]
- EUSTAR coauthors. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76:1327-39.
- [CrossRef] [PubMed] [Google Scholar]
- Superior mesenteric artery syndrome and intra-abdominal compartment syndrome in systemic lupus erythematosus. Lupus. 2014;23:194-6.
- [CrossRef] [PubMed] [Google Scholar]
- Superior mesenteric artery syndrome in a case of juvenile dermatomyositis: A unique complication. J Clin Rheumatol. 2021;27:S568-S570.
- [CrossRef] [PubMed] [Google Scholar]





