Translate this page into:
Guselkumab for psoriasis management in an HIV positive patient
Corresponding author: Dr. Lunfei Liu, Department of Dermatology, The Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, Zhejiang, China. 2197055@zju.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Qiu M, Feng B, Zhu C, Liu L. Guselkumab for psoriasis management in an HIV positive patient. Indian J Dermatol Venereol Leprol. 2025;91:115-6. doi: 10.25259/IJDVL_790_2024
Dear Editor,
Managing psoriatic disease in Human Immunodeficiency Virus (HIV) positive patients is particularly challenging due to the complexities of their immune system; however, the safety and efficacy of biologic agents in these patients are still uncertain. Here, we present the case of a 39-year-old Chinese male with a 23-year history of psoriasis, whose lesions worsened after HIV infection and were successfully controlled by guselkumab. Before the HIV diagnosis in 2018, his psoriasis symptoms could be improved by 95% with topical treatment (calcipotriol and corticosteroids ointment) and phototherapy. Despite being on combination antiretroviral therapy (ART) with lamivudine and dolutegravir, his previous therapy gradually lost its efficacy, resulting in papulosquamous plaques covering 43% of his body surface area. Apremilast was also found ineffective after three months of use. In June 2022, he made the first visit to our hospital and laboratory tests indicated hypertension, hyperlipidaemia, fatty liver, and hyperuricaemia, with no other significant abnormalities. His HIV infection was well managed with an undetectable viral load and a CD4 count of 590 cells/μL. After ruling out tumours, hepatitis B, and tuberculosis (TB), he was started on guselkumab, a biologic targeting IL-23, administered subcutaneously, 100 mg at week zero, week four, and every eight weeks thereafter. He continued using topicals for the first month but had stopped phototherapy and apremilast. Remarkable improvement was observed within two weeks, with significant improvement in itching and a reduction in PASI from 35.1 to 20.6. Over an eight-month period, the patient’s skin lesions almost completely resolved [Figure 1]. His CD4 count and HIV viral load remained stable and no adverse effects were observed indicating the combined treatment’s effectiveness and safety [Table 1]. To date, the patient has remained on guselkumab 100 mg every 8–12 weeks and has maintained complete clearance.
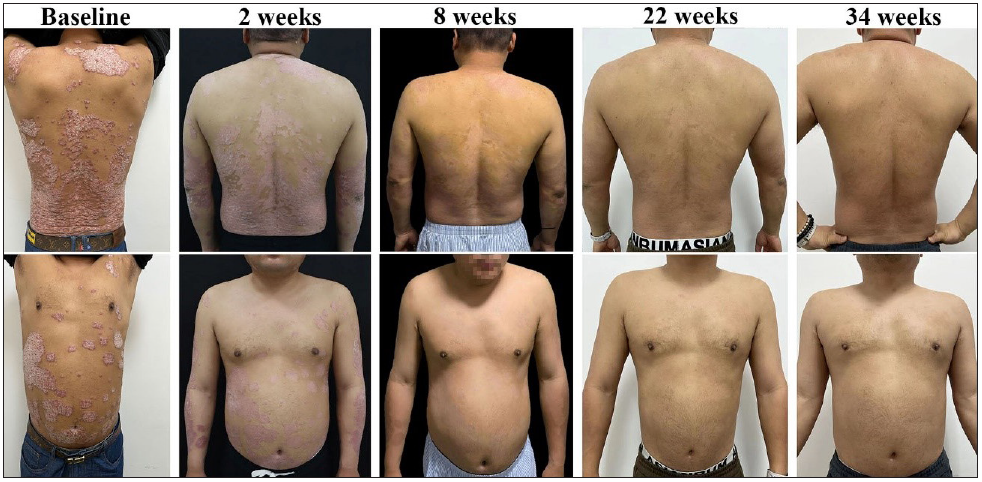
- Sequential lesion progression during guselkumab treatment.
| Duration of medication, weeks | 0 | 2 | 4 | 8 | 22 | 34 |
|---|---|---|---|---|---|---|
| Clinical Symptoms | ||||||
| PASI Score | 35.1 | 20.6 | 20 | 3.6 | 0.8 | 0 |
| PASI Score Decline Rate, % | / | 41.3 | 43 | 89.7 | 97.7 | 100 |
| BSA, % | 43 | 43 | 43 | 10 | 7 | 0 |
| PGA Score | 4 | 3 | 2 | 2 | 0 | 0 |
| Clinical indicators | ||||||
| CD4 T-lymphocyte count, cells/μL | 590 | 670 | / | 652 | 593 | / |
| HIV load, copies/mL | Undetectable | Undetectable | / | Undetectable | Undetectable | / |
PASI: psoriasis area and severity index, BSA: body surface area, PGA: physician’s global assessment
HIV infection can trigger or worsen psoriasis1 and treatment regimens for HIV-positive psoriasis are generally conservative to avoid destabilising the already compromised immune system. ART is the cornerstone therapy for people living with HIV (PLHIV) as it effectively suppresses viral replication and promotes immune system rebuilding. However, combination therapy with acitretin or apremilast is often necessary for PLHIV with moderate psoriasis. For those severe patients, psoriasis treatment guidelines suggest that biologics can be used with caution.2 Currently, the use of biologic agents is documented in case reports and lacks rigorous clinical trial data. A recent study by Sood et al. reviewed 132 HIV-positive patients with psoriasis who were treated with biologics, including TNF-α inhibitors, IL-17 inhibitors, and IL-23 inhibitors. The majority of patients experienced good clinical outcomes with the highest rates of complete clearance observed on IL-23 inhibitors and the most adverse effects, ranging from mild to fatal infections, associated with TNF-α inhibitors and IL-17 inhibitors.3 ART has improved the epidemiology and the survival rate of HIV, but opportunistic infections such as pneumocystis pneumonia, TB, and cryptococcal meningitis remain the most important causes of death in PLHIV.4 Extensive inhibition of TNF-α and IL-17 increases the risk of tumours and opportunistic infections. Given that China has one of the largest populations of patients with hepatitis B and TB, these two categories of drugs were not our preferred option. IL-23 induces IL-17A/F production by TH17 cells located further upstream of psoriasis pathogenesis and IL-23 inhibitors have demonstrated comparable efficacy and greater safety than IL-17 inhibitors in clinical application.5 The role of IL-23 in HIV invasion and CD4 cells depletion is not well documented. However, it is considered to be a crucial cytokine in those with poor reconstitution of CD4 T cells following ART,6 which means anti-IL-23 therapy may have a positive impact on increasing CD4 cells. Meanwhile, IL-23 inhibitors target the IL-23-p19 subunit, avoiding the inhibition of IL-12-mediated TH1 immune response,7 theoretically making them a suitable choice for psoriasis patients with HIV infection. Early and adequate ART can render HIV a manageable chronic condition, enabling patients with favourable immune profiles (CD4+ T-cell counts >200/cells/μL and stable viral loads) to consider biologics as a therapeutic option for managing psoriasis. While recent reports on IL-23 inhibitors primarily focus on risankizumab, our case suggests that combining ART with guselkumab therapy could also be an effective option for moderate to severe refractory psoriasis in HIV-infected patients who do not respond well to conventional treatments. However, regular follow-up is essential to monitor the adverse effects and changes in immunological profiles.
Ethical approval
The research/study was approved by the Institutional Review Board at the Institutional Ethics Review Board of Fourth Affiliated Hospital of Zhejiang University School of Medicine, number K2023113, dated 2023-07-10.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This research was supported by the Zhejiang Public Welfare Technology Research Project (Grant number: LGF20H110002) and the Zhejiang Provincial Natural Science Foundation of China (LQ20H110003).
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- British association of dermatologists guidelines for biologic therapy for psoriasis 2017. Br J Dermatol. 2017;177:628-36.
- [CrossRef] [PubMed] [Google Scholar]
- Use of biologic treatment in psoriasis patients with HIV: A systematic review. J Am Acad Dermatol 2024 S0190–9622(24)00442-0
- [CrossRef] [Google Scholar]
- A neglected epidemic: Fungal infections in HIV/AIDS. Trends Microbiol. 2014;22:120-27.
- [CrossRef] [PubMed] [Google Scholar]
- Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: A systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:1-25.
- [CrossRef] [Google Scholar]
- Link between interleukin-23 and anti-CD4 autoantibody production in antiretroviral-treated HIV-infected individuals. J Virol. 2021;95:e00271-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719-29.
- [CrossRef] [PubMed] [Google Scholar]





