Translate this page into:
Trichothiodystrophy in two siblings and utility of polarised transilluminating dermoscopy in its diagnosis
Corresponding authors: Dr. Kavita Bisherwal, Department of Dermatology and STD, University College of Medical Sciences and GTB Hospital, Delhi, India. kavita.bisherwal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Aggarwal B, Bisherwal K, Tyagi M, Singal A. Trichothiodystrophy in two siblings and utility of polarised transilluminating dermoscopy in its diagnosis. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_1146_2024
Dear Editor,
Trichothiodystrophy (TTD) is a rare autosomal recessive disorder associated with DNA (deoxyribonucleic acid) repair defect, caused by mutations in genes ERCC2, ERCC3 and GTF2H5. The acronyms PIBIDS, IBIDS and BIDS succinctly outline the six features: Photosensitivity, Ichthyosis, Brittle hair, Intellectual impairment, Decreased fertility, and Short stature.1 One of the defining characteristics of TTD is the presence of cysteine-deficient brittle hair, which can be elucidated through polarised light microscopy of hair shaft revealing distinctive alternating light and dark bands. Given the limited availability of polarised microscopy, utilisation of a simple handheld dermatoscope emerges as a practical alternative for visualising this characteristic banding pattern.2 We herein, present TTD in siblings, along with its heterogeneous presentation.
Two brothers aged eight and five years, born of consanguineous marriage, presented to the dermatology outpatients with generalised itching and dryness since birth along with short sparse hair over the scalp and eyebrows for one year of age. There was no history of sweating abnormality, difficulty in hearing or vision, photophobia, intellectual disability, recurrent infections or atopy. Gestational and birth history was uneventful. However, parents reported the presence of a parchment-like thick membrane in the elder child at birth, which shed off within two weeks with residual erythema and scaling. The younger child had only generalised erythema at birth. There was no developmental delay and both brothers had short stature on anthropometry. Examination revealed generalised xerosis with mild background erythema, more pronounced in photo-exposed areas with palmoplantar keratoderma and subacute eczematous lesions on the upper back. Diffuse alopecia with dry, coarse and brittle hair with prominent follicular papules were observed on the scalp [Figure 1a], eyelashes and eyebrows [Figure 1b]. The presence of striate leukonychia on fingernails was noted in the younger sibling. He also had non-palpable right-sided testes. Dental examination, intelligence quotient (IQ) evaluation and systemic examination were normal in both. Based on the history and examination, differential diagnoses of trichothiodystrophy and monilethrix were considered. Trichoscopic evaluation in both patients displayed decreased hair density, multiple yellow and white dots, trichoschisis (transverse fracture of the hair shaft) and multiple nodes at irregular intervals [Figure 2a and 2b]. The trichogram showed nodes at irregular intervals with an irregular hair cortex. On polarised transilluminating dermoscopy, distinct alternate dark and light bands were observed [Figure 3a]. Polarising microscopy also showed a similar banding pattern [Figure 3b]. Evaluations of haematological parameters, ophthalmology and ear-nose-throat examination and skeletal surveys revealed no abnormality. Ultrasonography revealed an absence of left testis in the scrotal sac in the younger sibling. Electron microscopy, biochemical and genetic testing for a conclusive diagnosis could not be done due to the non-availability of tests and monetary constraints. The collective clinical findings with trichoscopic evaluation led to the final diagnosis of trichothiodystrophy.
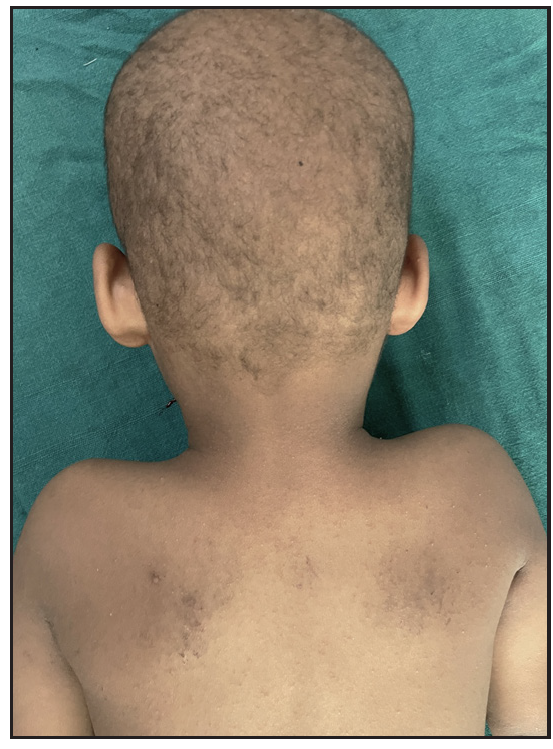
- Sparse brittle scalp hairs and eczematous lesions over the upper back.
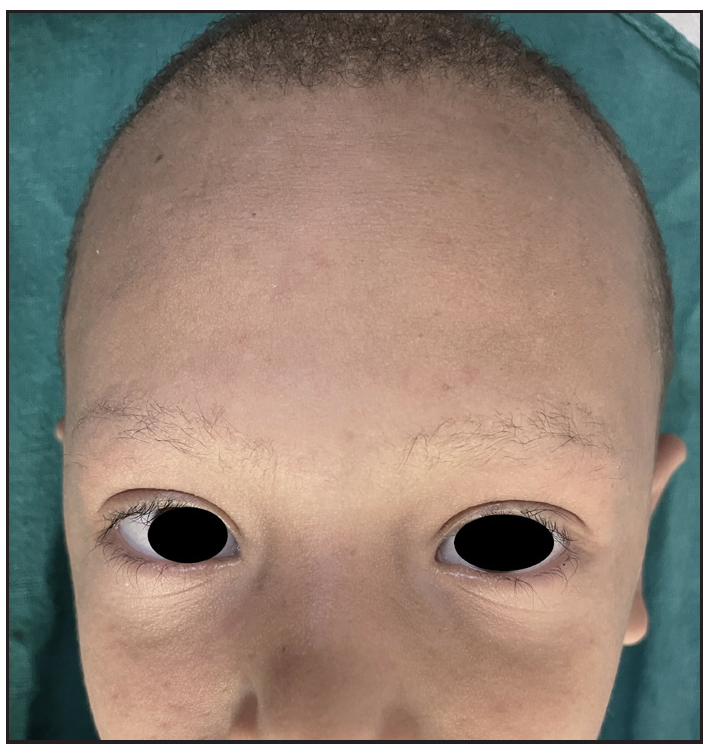
- Sparse eyebrows, eyelashes and scalp hair.
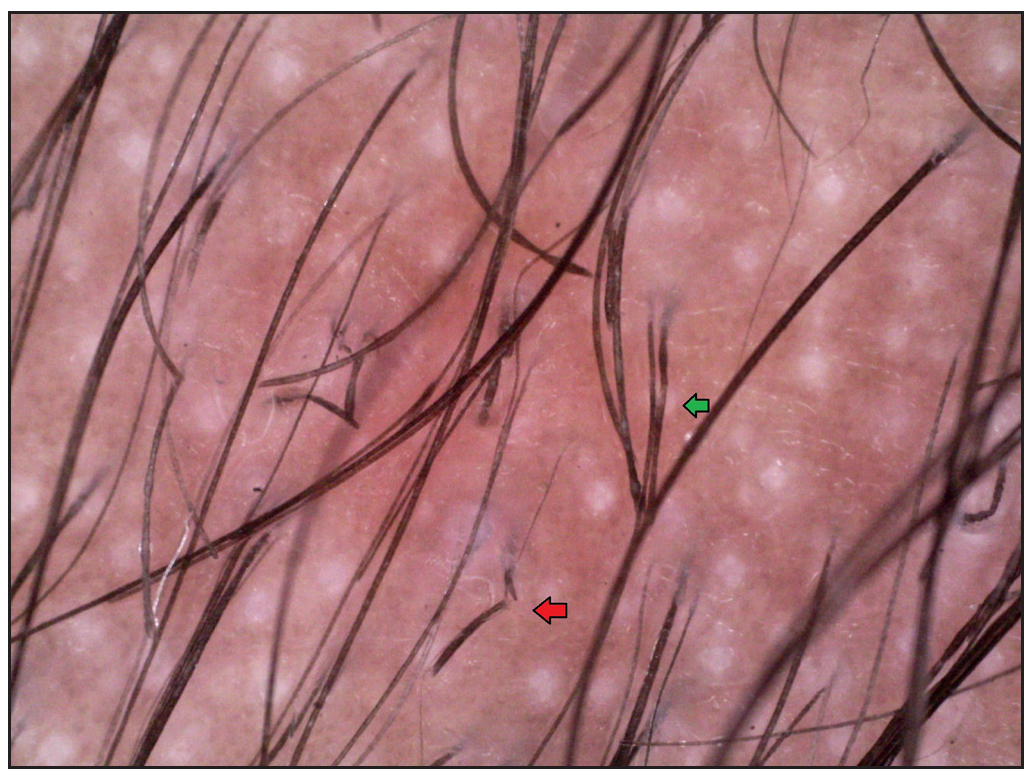
- Irregularly arranged multiple nodes (green arrow) and Trichoschisis (red arrow), white dots (Dino-lite AM7115MZT, non-contact, polarised mode, 50x).
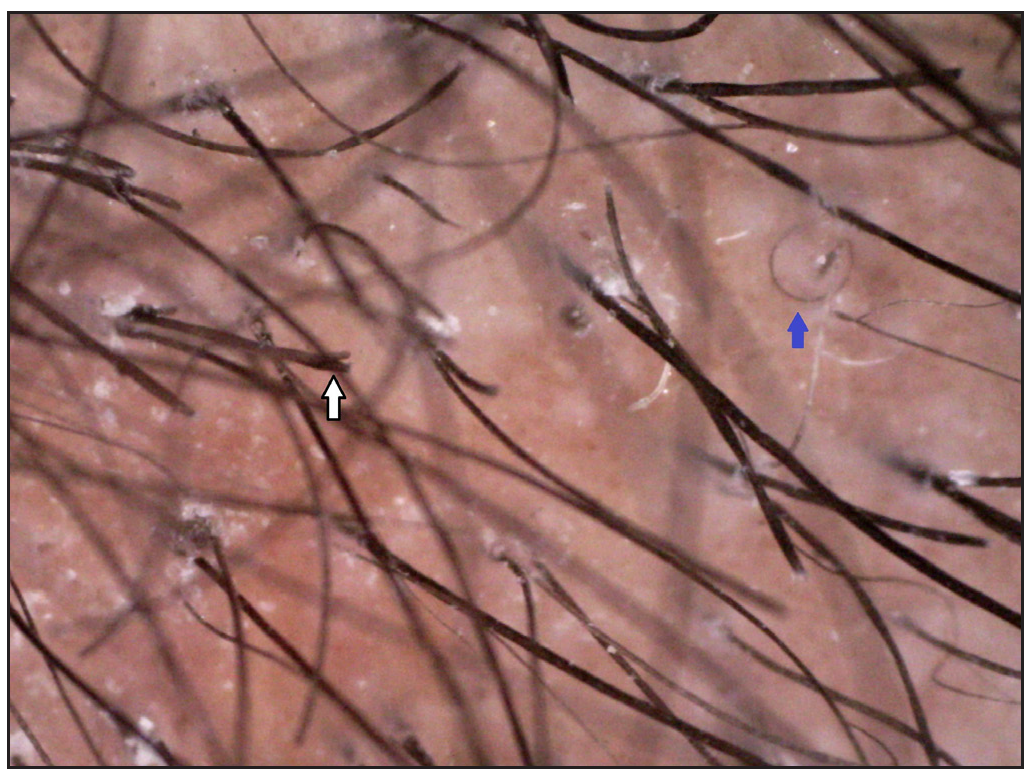
- Trichoptilosis (white arrow) and coiled hair (blue arrow) (Dino-lite AM7115MZT, non-contact, polarised mode, 50x).
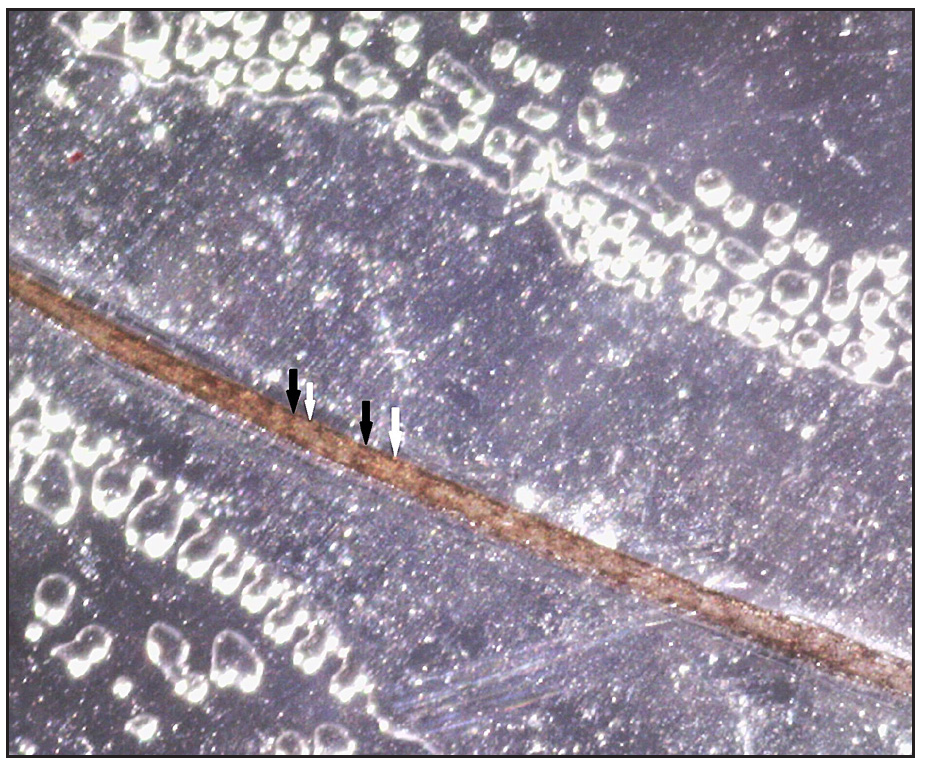
- Polarised transilluminating dermoscopy showing alternate dark (black arrows) and light band (white arrows) (Dino-lite AM7115MZT, non-contact, polarised mode, 50x).
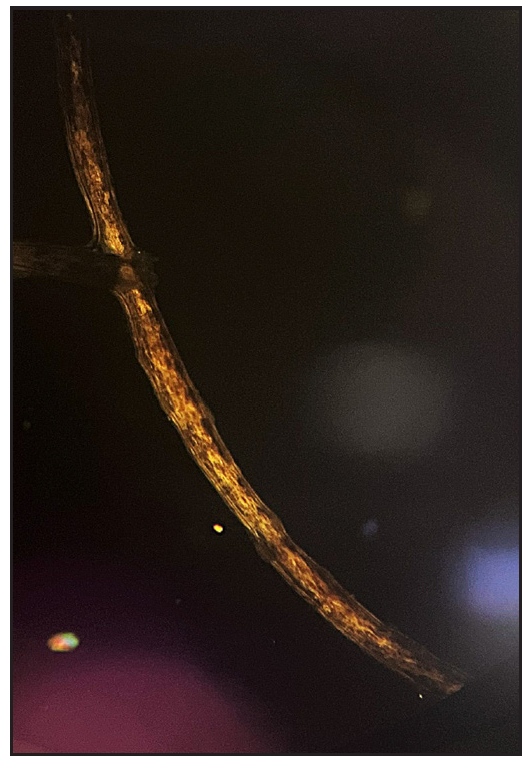
- Polarised microscopy (40x) showing alternate dark and light bands.
TTD is a multisystem disorder characterised by defective DNA repair and transcription mechanisms and presents in three primary types: photosensitive (mutations in ERCC2, ERCC3 or GTF2H), non-photosensitive (mutations in MPLKIP) and non-photosensitive with no identified genetic basis (no mutation encoding TTDN1).3 The reported incidence is approximately one per million live births and is rarely reported in siblings.4–7
TTD manifests a wide spectrum of clinical features ranging from mild disease with only brittle hair to severe disease with various neurological and developmental defects, recurrent infections and early mortality. Common associations include developmental delay/intellectual impairment (86%), short stature (73%), ichthyosis (65%), abnormal characteristics at birth (55%), ocular abnormalities (51%), infections (46%), photosensitivity (42%) and maternal pregnancy complications (28%).1
Though the definitive diagnosis of TTD is based on genetic analysis, hair shaft polarising microscopy (distinctive ‘tiger tail’ effect characterised by the regular undulation of the cortical hair fibre within the shaft) and biochemical analysis of hair (demonstrating low sulphur content in hair shaft) can also be employed.3 However, their use is restricted due to limited availability. Additionally, trichogram (revealing nodes) and trichoscopy (showing trichoschisis and nodes occurring at irregular intervals) can also aid in the diagnosis.
Yang et al. proposed polarised transilluminating dermoscopy with a light-emitting diode and mirror method as an alternative to polarising microscopy for the diagnosis of TTD.2 On a similar principle, we used double polarising dermoscopy as a simple diagnostic tool. Clipped hair were mounted on a glass slide and the polarised light was projected from below through a polarising dermatoscope (Dermlite DL-4). The specimen was then viewed through a polarised dermoscope placed above the glass slide (Dinolite AM7115MZT), which revealed a banding pattern. Management of TTD requires screening for systemic involvement, symptomatic management and counselling. Prenatal and newborn screening procedures are pivotal for early diagnosis, thereby enabling timely intervention.
The rare instance of two siblings with TTD without mental retardation underscores the diverse manifestations of the disease. Also, incorporating polarised transilluminating dermoscopy as a bedside tool can expedite the diagnosis in suspected cases of trichothiodystrophy specially in a resource-poor setting.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Trichothiodystrophy: A systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45:609-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Polarized transilluminating dermoscopy: Bedside trichoscopic diagnosis of trichothiodystrophy. Pediatr Dermatol. 2018;35:147-9.
- [CrossRef] [PubMed] [Google Scholar]
- Trichothiodystrophy: Update on the sulfur-deficient brittle hair syndromes. J Am Acad Dermatol. 2001;44:891-920. quiz 921–4
- [CrossRef] [PubMed] [Google Scholar]
- PIBIDS syndrome in two Brazilian siblings. BMJ Case Rep. 2018;11:e223744.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Trichothiodystrophy: Clinical spectrum, central nervous system imaging, and biochemical characterization of two siblings. J Invest Dermatol. 1994;103:154S-8S.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of trichothiodystrophy in 2 siblings. Dermatology. 1997;194:74-6.
- [CrossRef] [PubMed] [Google Scholar]
- ERCC2 mutations in two siblings with a severe trichothiodystrophy phenotype. J Eur Acad Dermatol Venereol. 2020;34:876-9.
- [CrossRef] [PubMed] [Google Scholar]





