Translate this page into:
Intradermal injection of botulinum toxin for erythema in rosacea: A scoping review and meta-analysis
Corresponding author: Dr. Yu-Chen Huang, Department of Dermatology, Wanfang Hospital, Taipei Medical University, Taipei, Taiwan. dhist2002@yahoo.com.tw
-
Received: ,
Accepted: ,
How to cite this article: Yeh MC-H, Shih Y-C, Huang Y-C. Intradermal injection of botulinum toxin for erythema in rosacea: A scoping review and meta-analysis. Indian J Dermatol Venereol Leprol. doi: 10.25259/IJDVL_274_2024
Abstract
Background
Rosacea is a skin condition characterised by persistent facial erythema, flushing, papules, pustules, and telangiectasia. Botulinum toxin A (BoNT-A) has been used to treat a variety of conditions, but its effectiveness in improving facial erythema in rosacea patients is uncertain.
Objectives
The aim of the study is to evaluate the effectiveness and determine the optimal dose of BoNT-A treatment for rosacea.
Methods
An online database search (Pubmed, Cochrane Library and Embase) was conducted on 30th June 2023 to identify studies that used intradermal injection of BoNT-A to treat facial erythema in rosacea patients and excluded studies in which BoNT-A was used for facial erythema due to other known medical condition such as menopause, drug or pregnancy. The primary outcome measure for this study was the improvement in erythema score as objectively assessed. A random effect model was used in the meta-analysis.
Results
Seven studies involving a total of 167 rosacea patients were included in the meta-analysis. Meta-analysis of two randomised controlled trials showed improvement of erythema on the third month after treatment standardized mean difference (SMD): 1.676, 95% confidence interval (CI): 2.278–1.074, I2: 35.76%). A separate analysis of seven single-armed treatment studies found significant improvement in erythema with intradermal injection of BoNT-A at one, two and three months after treatment (first month: SMD: 2.712, 95% CI: 4.1182–1.243; second month: SMD:2.213, 95% CI: 3.702–0.725; third month: SMD: 1.912, 95% CI: 2.882–0.941). Adverse events, including mild facial paralysis and injectional purpura, were reported in some studies.
Limitation
The limitations of this study include heterogeneity in study design and a small sample size.
Conclusion
Intradermal injection of BoNT-A may be an effective treatment for facial erythema in rosacea. Unwanted facial muscle paralysis was seen in different BoNT-A concentration but not noted when the dose was less than 0.02ml per site. Future studies particularly randomised trials are required to identify the volume of injection required to reduce the erythema
Keywords
Rosacea
botulinum toxin A
facial erythema
Introduction
Rosacea is a chronic relapsing inflammatory disorder involving the central face, characterised by persistent erythema, flushing, papules, pustules, telangiectasia, and even a stinging or burning sensation. Previous research has classified rosacea into four different subtypes1; however, due to the wide variety of presentation and overlapping symptoms, global consensus has evolved to a phenotype-based approach.2,3
Being one of the diagnostic phenotypes, centrofacial erythema is a frequent complaint in patients with rosacea. It can cause significant discomfort and cosmetic concerns. The burning sensation, papules, and pustules associated with erythema can be disturbing and may lead to feelings of self-consciousness or embarrassment.4 Previous studies showed erythema of the rosacea is associated with stigma, distress, anxiety, and depression.5 Such facial erythema often has remissions and exacerbations and can be challenging to manage. Various treatment options have been studied, including skin care and cosmetic treatments, topical therapies, oral therapies, laser and light-based therapies, injection therapies, and combination therapies. However, some cases of rosacea may be persistent and recalcitrant to treatment.
BoNT-A has been used for many years to treat a variety of dermatologic conditions, including hyperhidrosis and wrinkles.6,7 While it has not traditionally been used to treat rosacea, some studies have shown that it may be effective in improving facial erythema.8 Significant uncertainty remains about its efficacy, and the underlying mechanisms are not yet fully understood. Since botulinum toxin is often classified as a cosmetic treatment and typically not covered by national health insurance, it is crucial to evaluate its effectiveness critically. Therefore, this study aims to provide a comprehensive and quantitative evaluation of the current research on the use of BoNT-A in managing erythema in rosacea patients.
Methods
This meta-analysis was registered in the international prospective register of systematic reviews (PROSPERO, registration number CRD42022378394). The guide from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement, explanation and elaboration document, and checklist were used to present this study.
Data source and search strategy
Database (Pubmed, Cochrane Library and Embase) search was performed from inception to 30 January 2024. The search focused on clinical human studies in English. The keywords included the following: ‘botulinum toxin’ or ‘neurotoxin A’ combined with ‘rosacea’ or ‘erythematotelangietatic rosacea’ or ‘papulopustular rosacea’ and ‘rosacea erythema’. References of the screened articles were also included in the initial screening. The detailed search strategy is presented in Supplementary Table 1.
Eligibility criteria and study selection
The inclusion criteria for this study encompassed comparative investigations (randomised controlled trials/non-randomised controlled trials [RCTs/nRCTs]) that examined the treatment effects of intradermal BoNT-A in comparison to placebo. Additionally, studies that evaluated the combination of intradermal BoNT-A with another treatment for rosacea patients were considered eligible for inclusion. Furthermore, single-armed studies and case series focusing on intradermal BoNT-A monotherapy were also incorporated into the analysis. Review articles and conference reports were excluded.
In the existing literature, the terms ‘flushing’ and ‘erythema’ have been used interchangeably, leading to potential confusion. To ensure a comprehensive evaluation, we focused on studies that specifically addressed erythema and/or flushing in rosacea patients as well as reports involving patients with idiopathic persistent facial erythema, which is consistent with the phenotype of rosacea. Studies involving healthy populations – facial flushing caused by menopause, pregnancy, or other known medical conditions or using botulinum toxin other than type A – were excluded from our analysis.
Two independent investigators (MCHY and YCS) performed the initial screening of titles and abstracts to identify relevant articles, and subsequently, irrelevant publications were excluded from consideration.
Main outcomes
The principal objective of this research was to evaluate the efficacy of intradermal injection of BoNT-A in ameliorating erythema in rosacea. The timing of outcome assessment was up to three months after injection.
Quality assessment
The quality of the articles was evaluated independently using a methodological index for non-randomised studies (MINORS),9 which is a validated instrument to assess the methodological quality of non-randomised or non-comparative studies. It was originally designed for specialities in which randomised trials were difficult to conduct, such as surgical fields. It consists of 12 items, with the first eight tailored explicitly for non-comparative studies.
Data extraction
Two authors independently extracted the data and performed quality assessment (MCHY and YCS). Any disagreement between the two investigators was discussed and a consensus was reached. For each study, we extracted the study design, study country, sample size, patient population, treatment regimen, follow-up duration, and AEs. The extracted results are shown in Table 1, and the mean score and standard deviation of clinical erythema score for quantitative analysis are summarised in Supplementary Table 2. The assessment of erythema improvement was accomplished through a multifaceted approach, encompassing clinical evaluations conducted by qualified physicians, analysis of photographs or measurements obtained from specialised instruments such as a mexameter. If studies included both physician assessment and measurements from instruments, the results of physician assessment were used.
| Study | Country | Study Design | n. | Intervention group | Conc. [aliquot size] | Control group | Population | Symptoms | Follow up | Outcome assessment | Major AE | Minors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCTs | ||||||||||||
| Khoury et al.,15 2008 | USA | RCT | 14 (split-face) | IPL + Botox 8U per cheek | 1U/0.1ml [0.1ml] | IPL | Erythema or photoaging | erythema | 8 wks | CEA, fine wrinkles, hyperpigmentation | no | 19/24 |
| Dayan et al.,16 2017 | USA | RCT | 9 | Xeomin 10U per cheek | 1.4U/0.1ml | Saline | Rosacea | erythema | 20 wks | CEA, PSA, Mexameter | Not mentioned | 14/24 |
| Kim et al.,10 2019 | Korea | RCT |
23 (split-face) |
Nabota 15U per cheek | 1U/0.1ml [0.05ml] | saline | Rosacea | erythema | 8 wks | CEA, Global aesthetic improvement, biophysics* | no | 20/24 |
| Tong et al.,17 2021 | China | RCT | 22 (split-face) | BBL + Botox 10-15U per cheek | 1U/0.1ml [0.05ml] | BBL + saline | Rosacea | Erythema | 12wks | Mexameter | Mouth corner paralysis | 18/24 |
| Case series/Case reports | ||||||||||||
| Yuraitis et al.,18 2004 | USA | Case report | 1 | Botox 10U per cheek | 2U/0.1ml | - | Rosacea | Flushing | 4 wks | Visual improvment | No | 9/16 |
| Kranedonk et al.,19 2005 | USA | Case series | 1 | Botox 8U per cheek | 4U/0.1ml [0.05ml] | - | Rosacea | Erythema | 1 wk | Visual improvement | Cheek drop | 8/16 |
| Alexandroff et al.,20 2006 | UK | Case report | 2 | Botox 10U per cheek | 2U/0.1ml [0.1ml] | - | Not mentioned | Flushing | 6 wks | Visual improvement | Not mentioned | 9/16 |
| Dayan et al.,22 2012 | USA | Case series | 2 | Botox 8-12U per cheek | 1.4U/0.1ml | - | Rosacea | Flushing and erythema | 2 wks | Visual improvement | no | 9/16 |
| Bloom et al.,23 2015 | USA | Case series | 15 | Dysport 15-45U per cheek | 10U/0.1ml | - | Rosacea | erythema | 12 wks | CEA | No | 13/16 |
| Park et al.,24 2015 | Korea | Case series | 2 | Botox 5-15U per cheek | 2U/0.1ml | - | Rosacea | Flushing and erythema | 12 wks | Visual improvement | No | 9/16 |
| Park et al.,25 2018 | Korea | Case series | 17 | Meditoxin 10U per cheek | 2U/0.1ml | - | Rosacea | Recalcitrant erythema | 8 wks | Mexameter, PSA | Mild facial paralysis | 12/16 |
| Bharti et al.,26 2018 | India | Case report | 1 | 10U per cheek (brand not mentioned) | 1U/0.1ml [0.05ml] | - | Rosacea | Flushing and erythema | 16 wks | Visual improvement, dermoscopy | Not mentioned | 7/16 |
| Herane et al.,21 2020 | Chile | RCT |
18 (split-face) |
Botox 5U per cheek | 1U/0.1ml | Electroporation BTX | Rosacea | erythema | 12 wks | Erythema colorimetric scale | no | 19/24 |
| Al-Niaimi et al.,28 2020 | UK | Case series | 20 | PDL + Botox 10-20U injection or Dysport 20-50U per cheek |
Dysport 10U/0.1ml Botox 4U/ 0.1ml |
- | rosacea | Flushing and erythema | 52 wks | CEA | no | 13/16 |
| Luque et al.,29 2021 | Columbia | Case series | 3 | Dysport 10-15U or Xeomin 7 U per cheek |
Dysport 3.75U/0.1ml [0.02ml] Xeomin 1.25U/0.1ml [0.02ml] |
- | Rosacea | Flushing and erythema | 4 wks | Visual improvement | Not mentioned | 9/16 |
| Yang et al.,27 2022 | China | Case series | 16 | Hengli 20-30U per cheek | 1.6U/0.1ml [0.05ml] | - | erythema | Flushsing | 24 wks | CEA | Asymmetric facial expression | 13/16 |
| Calvisi et al.,30 2022 | Italy | Case series | 15 | Vistabex 10U per cheek |
2U/0.1ml [0.01ml] |
- | rosacea | erythema | 4 wks | DLQI, GAIS | no | 13/16 |
*Biophysics parameter: erythema index, melanin index, elasticity, hydration, Transepidermal water loss, RCT: Randomized controlled trial, IPL: intense pulsed light, CEA: clinical erythema assessment, PSA: patient subjective assessment, BTX: ****typo, please help change to botox, Nabota: ****another brand name for botumlinum toxin, BBL: broadband light, DLQI: Dermatology Life Quality Index, GAIS:Global Aesthetic Improvement Scale.
Statistical analysis
A random effects model was used for pooled estimates for all outcomes. To accommodate the different erythema scales from the studies, standardised mean difference (SMD) and 95% confidence interval were calculated. For heterogeneity across the studies, we used the I2 statistics. A threshold of > 50% was considered substantial. A p-value of < 0.05 was considered significant. Publication bias was not included as only less than ten RCTs were included in the study. The analysis was performed using the Comprehensive Meta-Analysis software version 3 (Biostat, Englewood, NK). Certainty of evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) criteria.
Results
Search results and trial characteristics
Initially, the literature search yielded 805 studies, from which 150 duplicates were removed. Seventeen studies were included in the systematic review and seven studies with 167 participants were found to meet the inclusion criteria for the meta-analysis. The PRISMA flow diagram is presented in Figure 1. It is worth noting that although four RCTs and 13 case series were included in the review, after stringent selection, only two RCTs and five case series entered quantitative analysis.
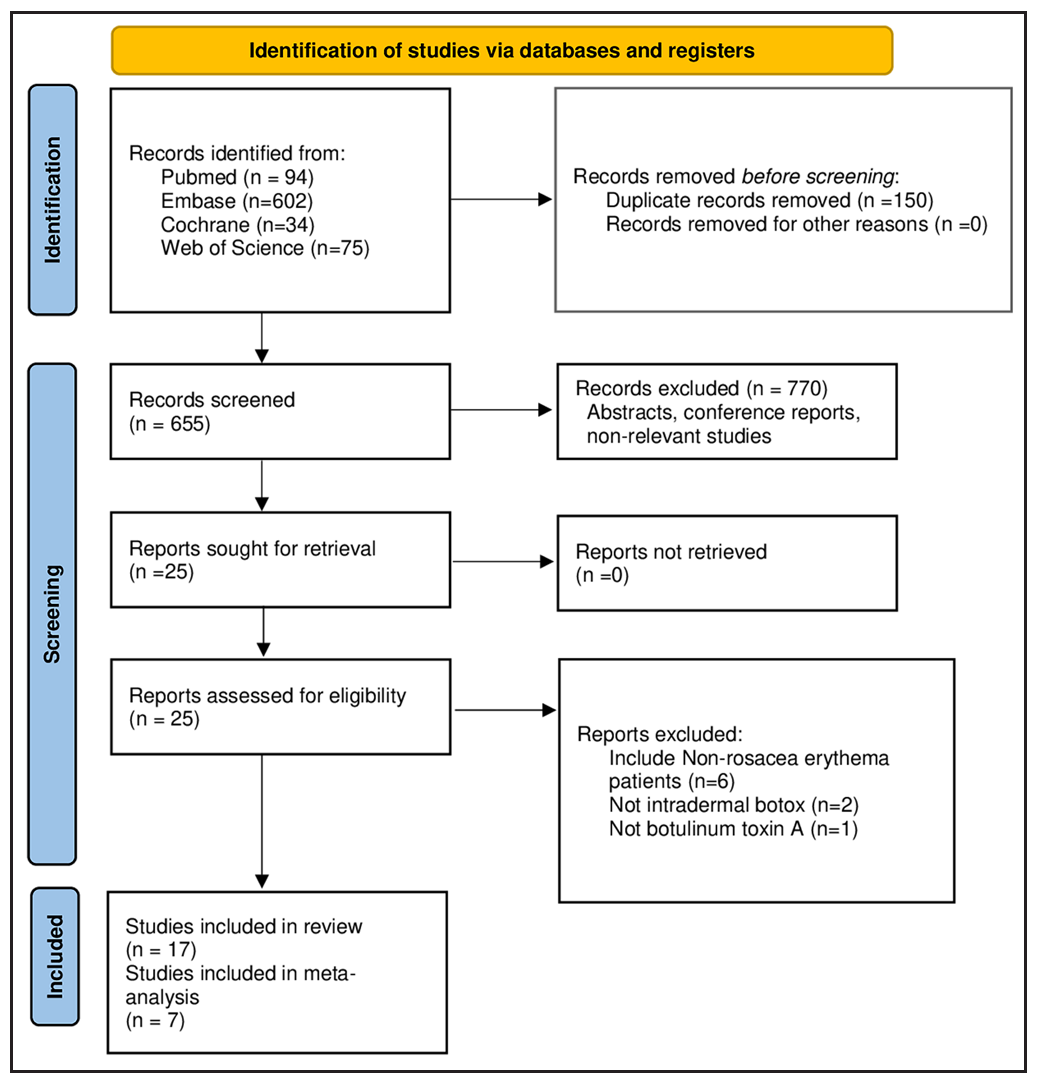
- The screening, inclusion and exclusion flow chart of the systematic review.
Quality assessment
Table 1 summarises the findings and the quality assessment of the included studies. For the four included RCTs, three RCTs were between 15 and 21, indicating a medium risk of bias. One RCT was scored 14, indicating a high risk of bias. Most randomised trials failed to report the inclusion of consecutive patients; only one study10 had a loss of follow-up rate less than 5% and none of the studies included a prospective calculation of the study size. The case series and case reports exhibited scores ranging from 7 to 13 out of 16, implying a medium to high risk of bias.
Characterisation of rosacea patients and botox use
The extracted clinical erythema scores ranging from ‒1.1 to 1.5 (negative denotes improvement of erythema) for meta-analysis are summarised in Supplementary Table 2. The utilisation of diverse BoNT-A products is from various companies. To enable an appropriate comparison between the different products, the units were standardised by converting them to the unit level of BOTOX. The studies also employed differing concentrations of BoNT-A, specifically 1U, 1.25U, 1.4U, 1.6U, 2U, 4U per 0.1 mL for the intradermal injections. Due to the requirement for multiple injections, the reported injection volumes varied, with quantities of 0.01, 0.02, 0.05, and 0.1 mL being documented.
Results of meta-analysis
The results of treatment from two RCTs, involving a total of 55 patients in split-faced trials, were subjected to quantitative analysis. The treatment’s effects did not show significance against the control during the initial and subsequent months (SMD for the first month: 3.386, 95% CI: 7.825–1.054, I2: 97.34%; SMD for the second month: 2.354, 95% CI: 5.492–0.784, I2: 96.51%). However, the treatment effect of BoNT-A became significant in the third month (SMD: 1.676, 95% CI: 2.278–1.074, I2: 35.76%) [Figure 2]. The GRADE evaluation rated the quality as moderate for the analysis of the first and second months and high for the third-month analysis [see Supplementary Table 3]. Other RCTs were reviewed, some of which utilised different result formats or presented incomplete findings. Khoury et al.15 assessed the potential adjunctive effect of intradermal BoNT-A combined with intense pulsed light therapy (IPL). While there appeared to be a tendency of greater improvement in erythema among patients who received both IPL and BoNT-A injection, the results did not show statistical significance. Dayan et al.16 conducted a trial against placebo saline injection; however, they only reported the reduction of the erythema score in the treatment arm. They observed a significant reduction in erythema starting from the first week, reaching its peak at the fourth week post-injection (‒1.4 ± 0.63, with the negative sign denoting a decrease in erythema compared to baseline). Scarcity of the RCTs was noted in this analysis, and future studies warrant more robust results.
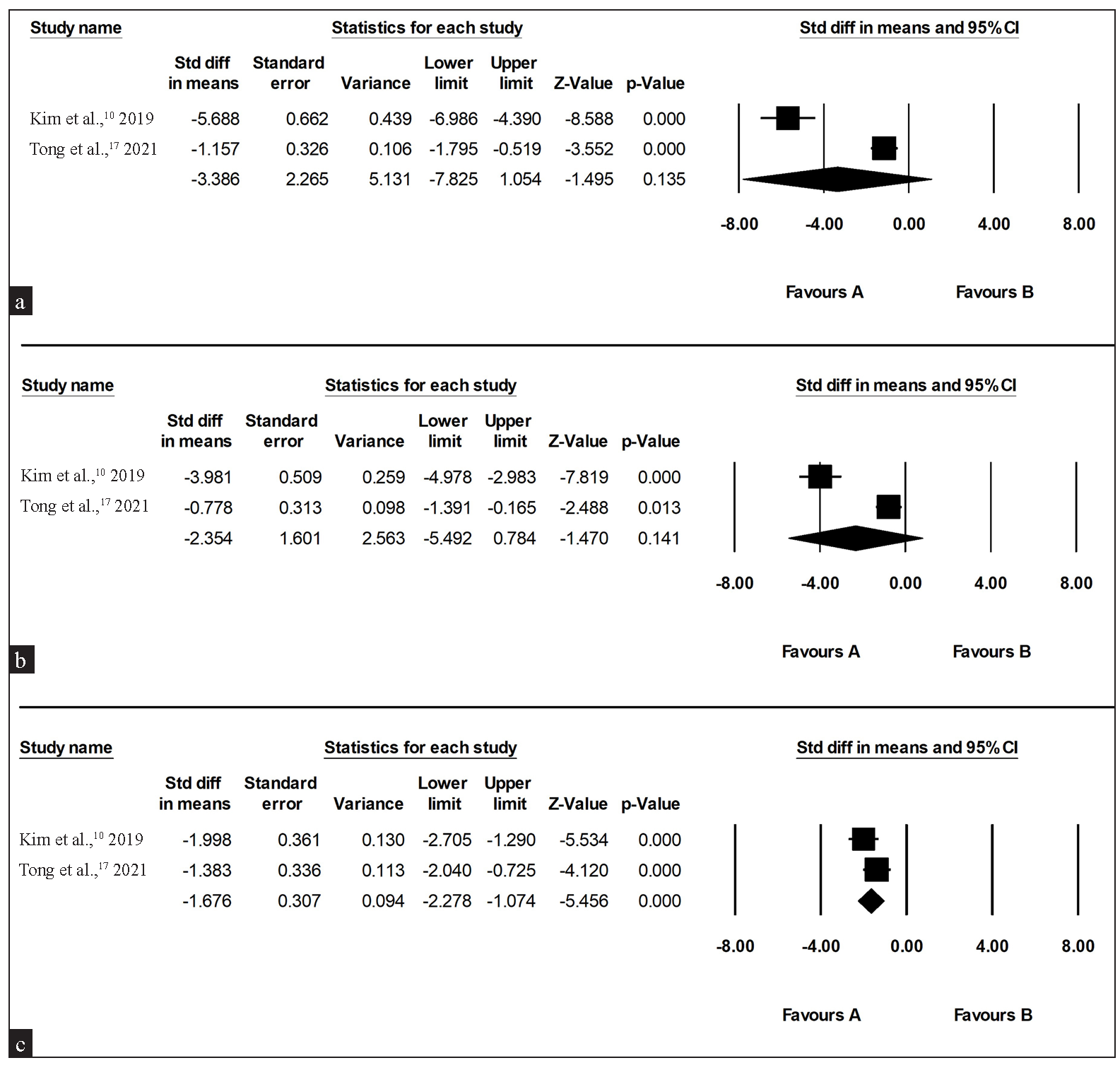
- Forest plot for adjunct intradermal botulinum toxin A against placebo (a) one month after treatment, (b) two months after treatment, (c) three months after treatment. (CI: confidence interval)
To gain a more comprehensive understanding of the pre-post treatment effect, seven studies with single-armed treatment of intradermal injection of BoNT-A for a total 93 patients were analysed. The treatment effect was significant in the first (SMD: 2.712, 95 % CI: 4.1182–1.243, I2: 89.529%), second (SMD: 2.213, 95% CI: 3.702–0.725, I2: 89.529%) and third month (SMD: 1.912, 95% CI: 2.882–0.941, I2: 78.219%) [Figure 3]. Although the treatment seemed effective, it is important to note that due to the high heterogeneity of the study, the GRADE evaluation for the quality of evidence was very low [Supplementary Table 3].
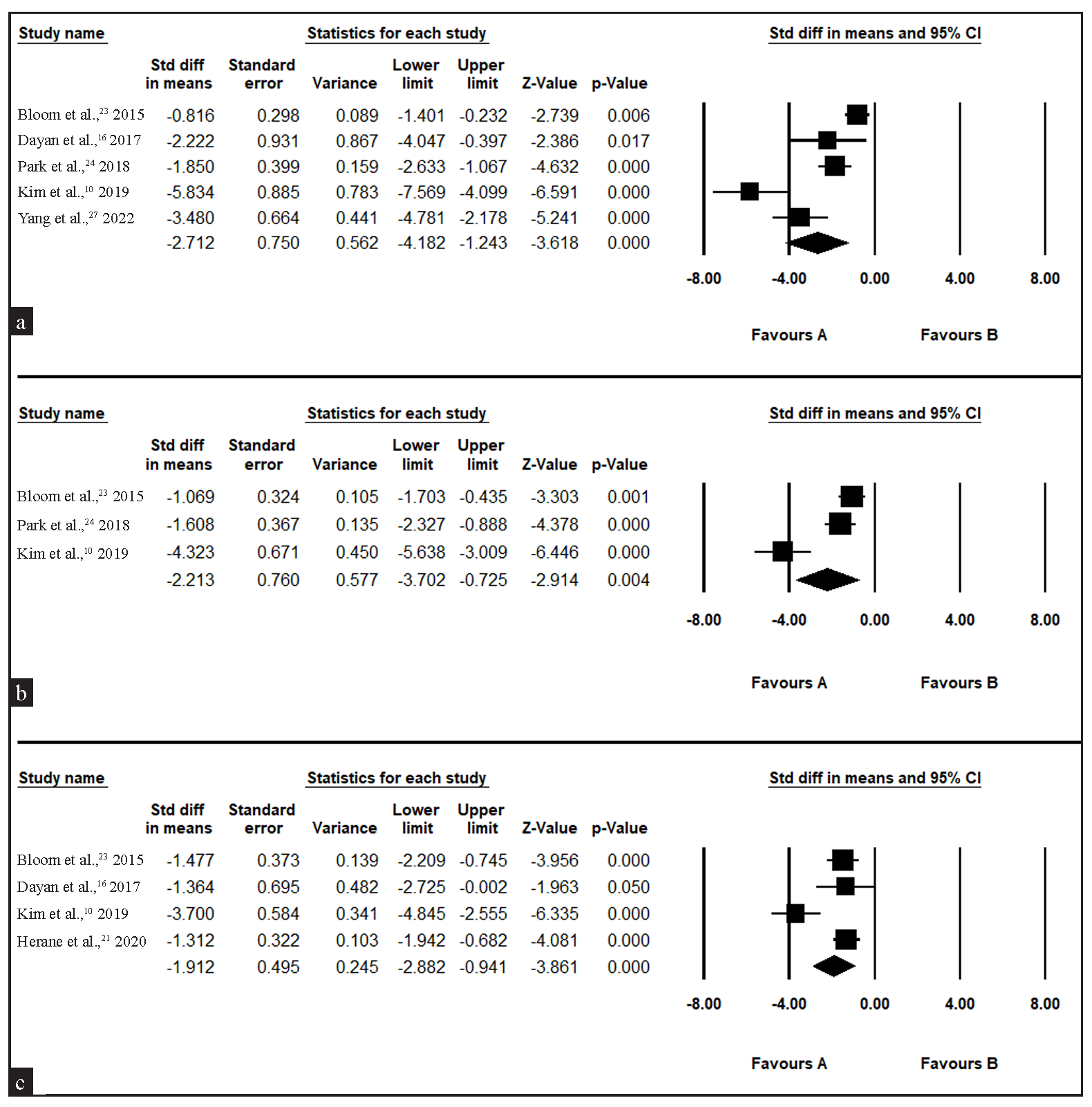
- Forest plot for single-armed intradermal botulinum toxin A for erythema in rosacea (a) one month after treatment, (b) two months after treatment, (c) three months after treatment. (CI: confidence interval)
Adverse events
Major AEs, including mild facial paralysis, asymmetric facial expression, mouth corner paralysis and cheek drop, and minor AEs, including injectional purpura and pain, were reported. It was worth noticing that facial muscle complication was reported in different toxin concentrations, including 4U, 2U, 1.6U, 1.4U, 1.25U and 1U per 0.1 mL (equivalent dose to onabotulinum toxin). In addition, facial muscle complications can occur in studies with single injection volume of 0.05 mL, but were not observed in studies with 0.02 or 0.01 mL [Table 1].
Discussion
This scoping review and meta-analysis has revealed that the intradermal injection of BoNT-A may represent a potential treatment option for facial erythema in rosacea patients. The strength of this study is the quantitative analysis at different time points after treatment. This could be used to guide clinical treatment and explanation.
Several plausible explanations could account for the observed effectiveness of BoNT-A in erythema treatment. One hypothesis postulates that the toxin may inhibit the expression of vascular endothelial growth factor by downregulating interleukin 8, resulting in reduced angiogenesis, persistent erythema and telangiectasia.11 Additionally, BoNT-A may influence the vasodilatory system by inhibiting the production of calcitonin gene-related peptide and substance P.12 Notably, the toxin has been found to reduce the expression of cyclooxygenase 2 and prostaglandin E2, thereby contributing to its anti-inflammatory effects and capacity to diminish facial erythema in rosacea patients.11,13
The effect of BoNT-A may also be associated with mast cell stability. Choi et al.14 conducted in vivo experiments to elucidate the underlying mechanism of BoNT-A in rosacea treatment. Their findings revealed that BoNT-A functions by impeding mast cell degranulation through cleavage of soluble N-ethylmaleimide-sensitive factor attachment protein receptor.. Consequently, the proposed action of BoNT-A involves targeting the neurogenic inflammatory aspect of rosacea while also exerting direct inhibitory effects on mast cells.
This meta-analysis revealed significant heterogeneity during the initial two months of treatment, likely stemming from the limited number of studies and variations in treatment response. Conversely, improvement in erythema was notably significant and consistent across studies in the third month, including those with single-armed designs. However, it’s noteworthy that results for the first and second months were inconsistent between RCTs and single-armed studies, underscoring the limitations of relying solely on case series for interpretation. Clinically, erythema can fluctuate and the placebo effect may introduce bias in single-armed designs, further emphasising the importance of rigorous study designs in assessing treatment efficacy.
Combining intradermal BoNT-A injection with other light-based treatments may be considered, but the results have shown variation. Although one randomised controlled trial has indicated greater improvement of erythema when combining broadband light with BoNT-A, the existing evidence is currently insufficient to make a definitive recommendation, and the underlying mechanism of the potential synergistic effect remains unclear.
One of the main side effects of BoNT-A treatment is unwanted muscle paralysis. This is particularly troublesome for treating erythema as a large area was needed for injection. To minimise this risk, many studies have used diluted concentrations and smaller injection volumes, resulting in the development of techniques such as microbotox and mesobotox. However, there is currently a lack of evidence on the optimal concentration and volume to achieve satisfactory results while avoiding facial muscle paralysis. Observations from included studies suggest that muscle paralysis can occur at concentrations ranging from 4U to 1U/0.1 mL and with injection volumes as small as 0.05 mL. Further research is needed to establish clinical guidelines for the optimal concentration and volume of BoNT-A for reducing facial erythema.
Limitation
The major limitation of this study is the high level of heterogeneity observed among the studies. The authors attempted to address this issue by calculating the SMD instead of the mean difference, but the differences in experimental methods, follow-up duration, treatment dosage and subjective evaluation methods may still contribute to the heterogeneity. Another limitation is the small number of included studies, as it can be challenging to conduct randomised trials in the field of cosmetic treatments. In some analyses, a trend was observed but did not reach statistical significance due to the limited sample size.
The heterogeneity of BoNT-A products and concentrations used in these investigations emphasises the necessity for a standardised approach in future research to enable accurate comparisons of outcomes across studies. Furthermore, the inclusion of various light-based therapies in the comparative analysis warrants careful consideration when interpreting the results. Future studies are required to address these concerns and enhance the overall comprehension of the therapeutic potential of BoNT-A within the context of intradermal administration.
Conclusion
This study suggests that the intradermal administration of BoNT-A may be an effective therapeutic option for managing rosacea-associated erythema. Nevertheless, further investigation about to rosacea and BoNT-A is needed.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Standard classification of rosacea: Report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol. 2002;46:584-87.
- [CrossRef] [PubMed] [Google Scholar]
- Standard classification and pathophysiology of rosacea: The 2017 update by the national rosacea society expert committee. J Am Acad Dermatol. 2018;78:148-55.
- [CrossRef] [PubMed] [Google Scholar]
- Updating the diagnosis, classification and assessment of rosacea: Recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431-8.
- [CrossRef] [PubMed] [Google Scholar]
- Feelings of stigmatization in patients with rosacea. J Eur Acad Dermatol Venereol. 2017;31:163-6.
- [CrossRef] [PubMed] [Google Scholar]
- Symptom severity and psychological sequelae in rosacea: Results of a survey. Psychol Health Med. 2014;19:586-91.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment approaches and outcomes associated with the use of abobotulinumtoxinA for the treatment of hyperhidrosis: A systematic review. J Am Acad Dermatol. 2021;85:1121-9.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin in the field of dermatology: Novel indications. Toxins (Basel). 2017;9:403.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of botulinum toxin in treating rosacea: A systematic review. Clin Cosmet Investig Dermatol. 2021;14:407-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J Surg. 2003;73:712-6.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of skin physiology change and safety after intradermal injections with botulinum toxin: A randomized, double-blind, placebo-controlled, split-face pilot study in rosacea patients with facial erythema. Dermatol Surg. 2019;45:1155-62.
- [CrossRef] [PubMed] [Google Scholar]
- Down regulation of vascular endothelial growth factor is associated with decreased inflammation after intravesical Onabotulinumtoxin A injections combined with hydrodistention for patients with interstitial cystitis—clinical results and immunohistochemistry analysis. Urology. 2013;82:1452.e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Neuropeptide control mechanisms in cutaneous biology: Physiological and clinical significance. J Invest Dermatol. 2006;126:1937-47.
- [CrossRef] [PubMed] [Google Scholar]
- Intravesical botulinum toxin A administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol. 2009;56:159-66.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J Dermatol Sci. 2019;93:58-64.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effect of botulinum toxin type A on full-face intense pulsed light treatment: A randomized, double-blind, split-face study. Dermatol Surg. 2008;34:1062-9.
- [CrossRef] [PubMed] [Google Scholar]
- A Pilot, double-blind, placebo-controlled study to assess the efficacy and safety of incobotulinumtoxinA injections in the treatment of rosacea. J Drugs Dermatol. 2017;16:549-54.
- [PubMed] [Google Scholar]
- A randomized, controlled, split-face study of botulinum toxin and broadband light for the treatment of erythematotelangiectatic rosacea. Dermatol Ther. 2022;35:e15395.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin for the treatment of facial flushing. Dermatol Surg. 2004;30:102-04.
- [CrossRef] [PubMed] [Google Scholar]
- Re: Botulinum toxin for the treatment of facial flushing. Dermatol Surg. 2005;31:491. author reply 492
- [CrossRef] [PubMed] [Google Scholar]
- Successful use of botulinum toxin a for the treatment of neck and anterior chest wall flushing. Dermatol Surg. 2006;32:1536.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin for the treatment of erythema and flushing of rosacea with two different techniques: Intradermal injections and facial electroporation. Surg Cosmet Dermatol. 2020;12:326-31.
- [CrossRef] [Google Scholar]
- A new treatment regimen for rosacea: OnabotulinumtoxinA. J Drugs Dermatol. 2012;11:e76-9.
- [PubMed] [Google Scholar]
- Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol Surg. 2015;41(Suppl 1):S9-16.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology. 2015;230:299-301.
- [CrossRef] [PubMed] [Google Scholar]
- A pilot study to evaluate the efficacy and safety of treatment with botulinum toxin in patients with recalcitrant and persistent erythematotelangiectatic rosacea. Ann Dermatol. 2018;30:688-93.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Mesotherapy with botulinum toxin for the treatment of refractory vascular and papulopustular rosacea. J Am Acad Dermatol. 2023;88:e295-6.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin A alleviates persistent erythema and flushing in patients with erythema telangiectasia rosacea. Dermatol Ther (Heidelb). 2022;12:2285-94.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pulsed dye laser followed by intradermal botulinum toxin type-A in the treatment of rosacea-associated erythema and flushing. Dermatol Ther. 2020;33:e13976.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin: An effective treatment for flushing and persistent erythema in rosacea. J Clin Aesthet Dermatol. 2021;14:42-5.
- [PubMed] [PubMed Central] [Google Scholar]
- Microbotox: A prospective evaluation of dermatological improvement in patients with mild-to-moderate acne and erythematotelangiectatic rosacea. J Cosmet Dermatol. 2022;21:3747-53.
- [CrossRef] [PubMed] [Google Scholar]






