Translate this page into:
Nailfold capillaroscopy with USB dermatoscope: A cross-sectional study in healthy adults
Correspondence Address:
Chander Grover
Department of Dermatology and STD, University College of Medical Sciences, Delhi - 110 095
India
| How to cite this article: Jakhar D, Grover C, Singal A. Nailfold capillaroscopy with USB dermatoscope: A cross-sectional study in healthy adults. Indian J Dermatol Venereol Leprol 2020;86:33-38 |
Abstract
Background: Nailfold capillaroscopy (NFC) is a convenient method for studying capillary morphology in the proximal nailfold (PNF) and is used for the evaluation of connective tissue and other diseases affecting the microvasculature. However, capillary density and morphological patterns in healthy individuals are largely unknown and this compromises the evaluation of the microvasculature in disease states.
Objective: To describe and quantify the morphological characteristics of nailfold capillaries in healthy adult Indians.
Methods: A USB 2.0 dermatoscope (Dinolite AM413ZT) with polarizing light was used to study nailfold capillary characteristics in 50 consecutive healthy adult individuals. NFC was performed on all 10 fingers. Images were assessed for both quantitative and qualitative features.
Results: The mean capillary density in healthy Indian adults was 7.63 ± 1.12 capillary/mm. Tortuosity (22%), meandering capillaries (14%) and microhemorrhages (14%) were frequently seen in these individuals.
Limitation: The small sample size limited a conclusive determination of statistically significant differences in NFC findings with respect to gender and age.
Conclusion: NFC with a USB dermatoscope is a useful technique for studying the PNF capillaries. The normal PNF capillary density in healthy Indian adults was 7.63 ± 1.12 capillary/mm. Capillary alterations such as tortuosity, meandering capillaries and microhemorrhages are seen in a significant number of healthy individuals.
Introduction
Nailfold capillaroscopy (NFC) is a reproducible, noninvasive, painless, and inexpensive technique for studying proximal nailfold (PNF) capillaries.[1] NFC was initially performed with a magnifying lens and later with wide-field microscopes and ophthalmoscopes.[2],[3] The current generation of dermatoscopes and videocapillaroscopes provide high magnification (~200×) and polarization and have sophisticated software simplifying the study of capillary morphology. These newer devices also have real-time control of image capture, storage and analysis, and inbuilt software to measure dimensions in millimeter (mm).[2]
The interpretation of NFC data is restricted by the paucity of studies in normal individuals. NFC findings may be influenced by age, ethnicity, geographic region, inter-observer variation and differences in skin transparency (owing to skin pigmentation, hyperkeratosis, injuries, edema, etc.).[2],[4],[5],[6],[7],[8],[9],[10] The devices used, magnification employed and the use of different methods to calculate the mean capillary density may also influence NFC findings. Panoramic NFC and wide-field capillary microscopy have been used in the past to define abnormal capillary morphology and capillary density[3],[10] but the high magnifications employed by the current generation USB dermatoscopes makes it mandatory to use standardized and reproducible methodology.
Most available studies are from the Caucasian population[2],[4],[5],[6],[7],[8],[10] and it would be imprudent to extrapolate Caucasian data to the Indian skin owing to innate differences. This study was designed, therefore, to qualitatively and quantitatively describe normal nailfold capillary morphology in the healthy adult Indian population.
Methods
This observational, analytical study was conducted in the Department of Dermatology and STD at University College of Medical Sciences and GTB Hospital, New Delhi, India. The Institutional Review Board and Ethical Committee approved the study protocol. Fifty healthy adult volunteers (above 18 years) accompanying patients visiting the dermatology outpatient clinic were included in the study. Individuals with a systemic disease or on medications affecting the peripheral microcirculation, and pregnant and lactating females were excluded from the study. Those with a history or clinical evidence of smoking, onychophagia, onychotillomania, trauma, nail infections and recent manicure were also excluded.[2] A detailed clinical examination of all 20 nails was performed in all study subjects.
Each volunteer was made to sit comfortably at an ambient room temperature for 15 min, and then subjected to a detailed NFC on all 10 fingernails with the hands placed on a dull non-refractile surface at the level of heart. The polarizing mode of a USB 2.0 videodermatoscope (Dinolite AM413ZT; 20–220×; 1.3 MP) was used both with and without linkage fluid (immersion oil). NFC was performed by a single observer (DJ) first at low magnification (50×) to provide a global evaluation and then at higher magnification (200×) for assessing the detailed morphology of the capillaries. The images were stored, processed and interpreted by two independent observers.
The following NFC parameters were recorded:
Quantitative parameters
The NFC density was calculated as the number of distal most capillary loops in the fourth and fifth fingers of both the hands.[2] A mark was carefully made with an ultra-thin marker pen at the center of the PNF near the distal end of cuticle (so as to avoid obscuring any capillaries) and then an image (at high magnification) was captured on either side of the mark. Both these images were analyzed with the calibration software of the USB dermatoscope, counting the distal most capillary loops visible over a 2 mm length on either side of the marked point [Figure - 1]a and [Figure - 1]b. This gave the number of capillaries over a 4 mm length in an individual finger. The number of capillary loops of four fingers (right and left fourth and fifth fingers) in each volunteer were added and the sum was divided by 16 to calculate mean capillary density/mm. The mean capillary density for all 50 volunteers was thus recorded.
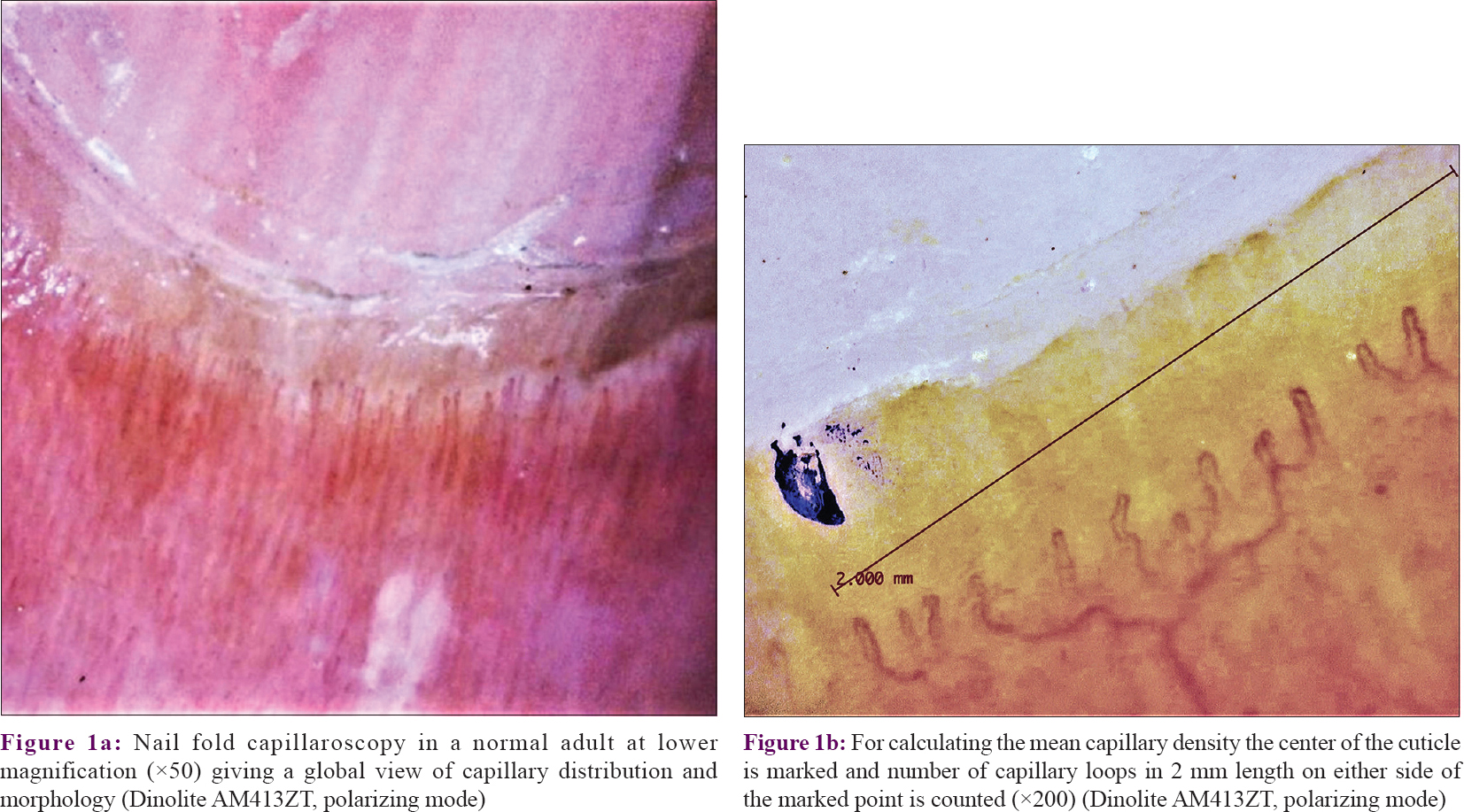 |
| Figure 1 |
Qualitative parameters
The various morphological alterations in the capillary loops (described in [Table - 1]) were carefully visualized and recorded. The degree of capillary loss was evaluated on a scale proposed by Lee et al.[11] The visibility of the subpapillary plexus was recorded as present or absent. An abnormal capillary architecture was considered to be present in an individual when it was seen in more than two fingers.
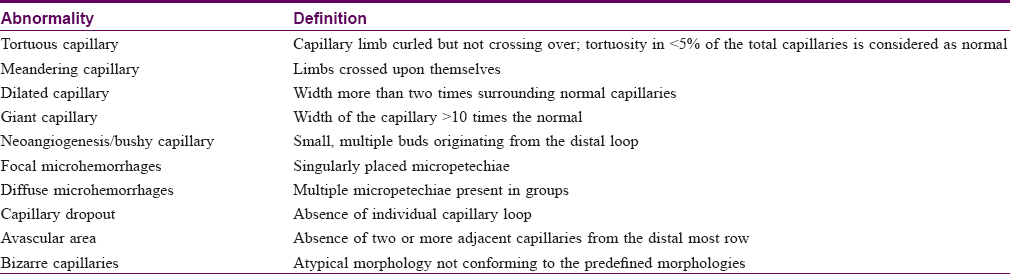
Statistical analysis
Both the qualitative and quantitative data were recorded and analyzed using SPSS version 20. The Mann–Whitney test was applied for quantitative parameters, whereas the Pearson χ[2]-test was used for studying the possible relationships between qualitative parameters and other variables such as age and gender. A p value <0.05 was considered significant.
Results
Fifty healthy adult volunteers (29 female, 21 male) were selected for the study. Their ages ranged from 24 to 70 years (mean = 44.26 ± 15.14 years) and the mean body mass index was 23.88 ± 3.35. Clinical examination of the nails revealed longitudinal ridging in 14 (28%), ragged cuticles in 13 (26%) and punctate leukonychia in 14% of these subjects. Pitting (12%), onychoschizia (12%), Beau's lines (10%), longitudinal melanonychia (6%) and nail plate discoloration (4%) were also observed.
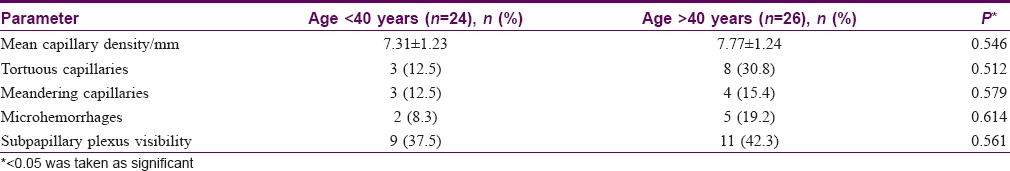
The mean capillary density in our subjects was 7.63 ± 1.12 capillary/mm (range = 6–10 capillary/mm) [Table - 2]. The mean capillary density was higher in females (7.64 ± 1.23 vs 7.43 ± 1.29 capillary/mm) but the difference was not statistically significant (P = 0.653). The capillary density did not vary significantly with age (P = 0.546) [Table - 3]. Tortuous capillaries (22%), microhemorrhages (14%), meandering capillaries (14%), dilated capillaries (6%), bushy capillaries (4%) and bizarre capillaries (2%) were all noted [Figure - 2]a, [Figure - 2]b, [Figure - 2]c, [Figure - 2]d but capillary dropouts and avascular areas were not seen. The subpapillary plexus was visible in 40% of the volunteers. There were no significant differences in qualitative features between the sexes. The incidence of capillary tortuosity increased with age but the difference was not statistically significant (P = 0.512) [Table - 3].
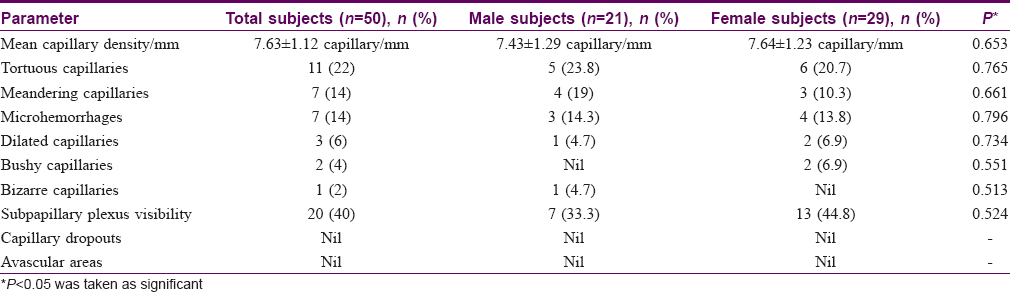
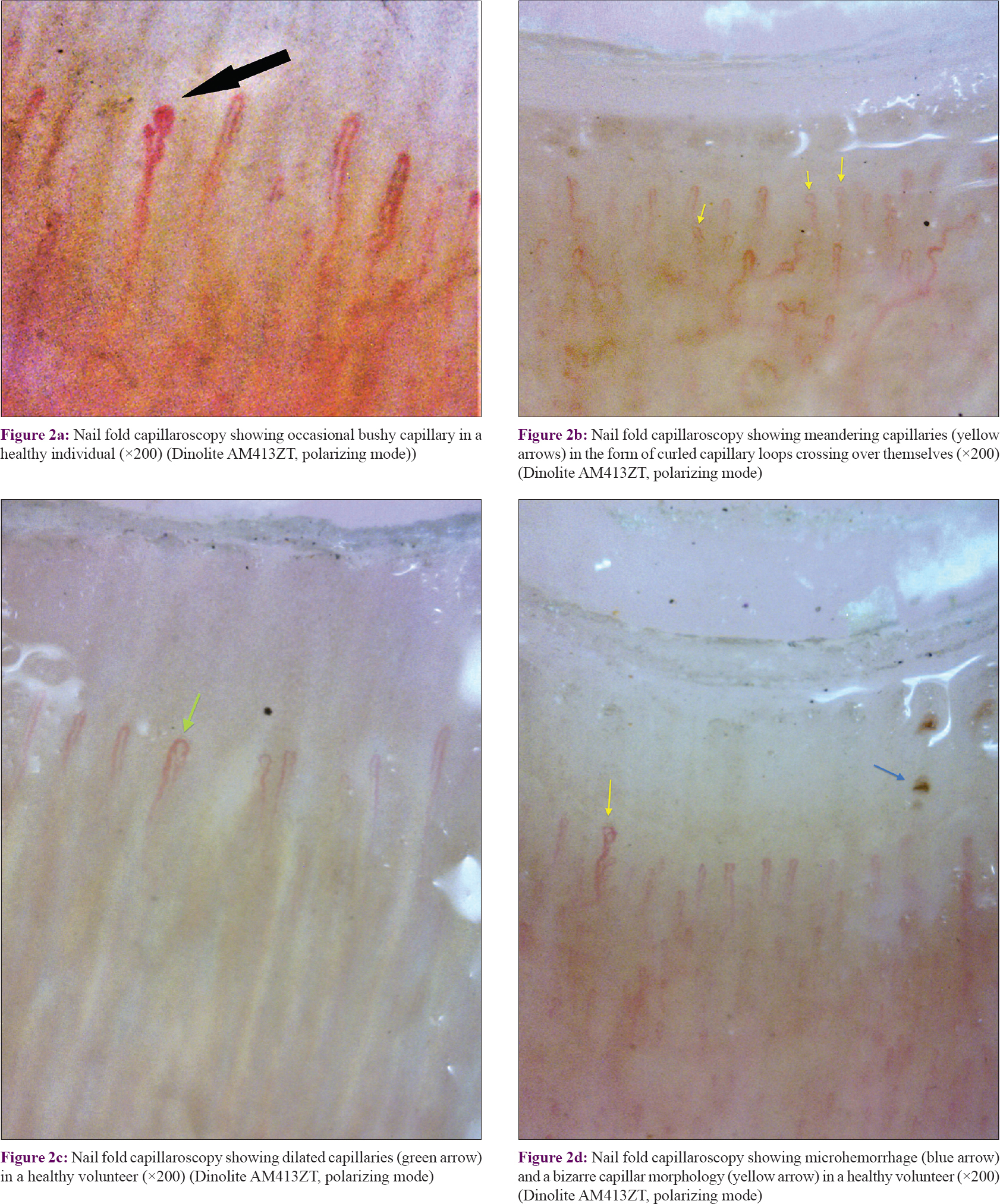 |
| Figure 2: |
Discussion
Since Johan Christophorous Kolhaus first observed nailfold capillaries nearly 4 centuries ago with a primitive microscope, NFC has become a standard evaluation technique in the fields of dermatology and rheumatology.[12],[13],[14],[15],[16],[17] The capillaries in the PNFs run parallel to the skin surface with the distal row being entirely visible throughout its length, appearing as hairpin-shaped loops with an afferent limb, an apical turn and an efferent limb.[13]
Nailfold capillary parameters were evaluated both quantitatively and qualitatively in normal, healthy volunteers using an objective and reproducible method to minimize subjectivity. The influence of factors such as age and gender was explored separately for each parameter. Although ideally all the finger nailfolds should be screened, in most studies quantitative assessment has been done only on the fourth and fifth fingers, and qualitative assessment in 2–4 fingers (excluding the thumb).[3] We performed NFC on all fingers. The maximal visibility of nailfold capillaries was found to be comparable in fourth and fifth finger, whereas it was minimal in the thumb. Owing to the convexity of the proximal nailfold only the central portion remains focused at higher magnifications (200×) while the periphery usually goes out of focus and it is not possible to count beyond 2 mm from the centre. Therefore we marked the center of the cuticle with a thin-pointed marker pen and evaluated and counted the capillaries up to 2 mm either side of the mark. This method is easy to perform and reproducible.
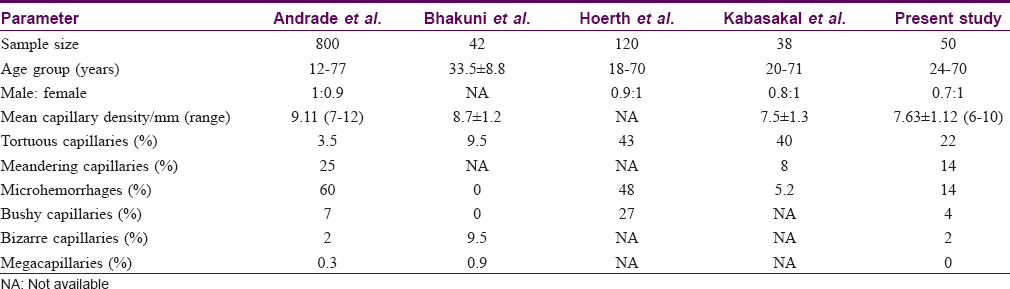
The mean capillary density in the present study was 7.63 ± 1.12/mm (range of 6–10 capillary/mm). Previous studies have reported mean capillary densities varying from 7 to 14 capillaries/mm in different ethnic groups [Table - 4].[5],[9],[10],[18] The mean capillary density in our study group did not significantly vary with age or gender, which is in agreement to previous studies.[2],[5],[10]
Although earlier studies using wide-field capillaroscopy found a low prevalence of morphologically abnormal capillaries in healthy individuals,[3] these were much more frequent in our study. Tortuosity was the most common qualitative abnormality observed as noted in earlier studies.[3],[5],[17],[18] There was no significant variation in the incidence of tortuosity with age or gender. At higher magnifications some degree of tortuosity is visible in most capillaries and we suggest that tortuosity should be considered significant only if present along the entire length of both the afferent and efferent loops.
Meandering capillaries were the dominant morphological abnormality encountered by Andrade et al.[10] and these were also common in our subjects. Microhemorrhages were also frequently seen and were focal in all the subjects. No subject demonstrated extensive or multiple microhemorrhages. Taken together with other reports,[9],[10] our findings suggest that focal micropetechiae may result from micro-trauma inflicted during normal daily routines, whereas diffuse microhemorrhages suggest an endogenous endothelial injury indicating a microangiopathy.[3]
Dilation of capillaries was uncommon (3/50) and giant capillaries were not seen. Dilation generally represents the first sign of micro-vessel injury and presence of even a single giant capillary has been considered to be a potential marker of microangiopathy.[17] Absence of giant capillaries and capillary dropouts in our study subjects was consistent with previous reports.[2],[5],[9],[10]
The subpapillary plexus was visible in 40% of the subjects and the visibility was highest in the fourth and fifth fingers. The visibility of the subpapillary plexus shows considerable variablity between studies[2],[5],[10] attributable to differences in resolution, magnification and polarization of the dermatoscope as compared to the wide-field microscopy done in earlier studies.
Limitations
The small sample size limits conclusive correlation of the influence of age and gender on NFC findings.
Conclusion
A standardized technique of performing NFC using a USB dermatoscope is described. NFC parameters and morphological abnormalities in the capillaries in the normal adult Indian population are highlighted.
Declaration of patient consent
The authors certify that they have obtained all appropriate volunteers consent forms. In the form, the volunteers have given their consent for their images and other clinical information to be reported in the journal. The volunteers understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Shore AC. Capillaroscopy and the measurement of capillary pressure. Br J Clin Pharmacol. 2000;50:501-13.
[Google Scholar]
|
| 2. |
Ingegnoli F, Gualtierotti R, Lubatti C, Bertolazzi C, Gutierrez M, Boracchi P, et al. Nailfold capillary patterns in healthy subjects: A real issue in capillaroscopy. Microvasc Res. 2013;90:90-5.
[Google Scholar]
|
| 3. |
Maricq HR. Widefield capillary microscopy: Technique and rating scale for abnormalities seen in scleroderma and related disorders. Arthritis Rheum. 1981;24:1159-65.
[Google Scholar]
|
| 4. |
Ingegnoli F, Zeni S, Gerloni V, Fantini F. Capillaroscopic observations in childhood rheumatic diseases and healthy controls. Clin Exp Rheumatol. 2005;23:905-11.
[Google Scholar]
|
| 5. |
Kabasakal Y, Elvins DM, Ring EF, McHugh NJ. Quantitative nailfold capillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann Rheum Dis. 1996;55:507-12.
[Google Scholar]
|
| 6. |
Dolezalova P, Young SP, Bacon PA, Southwood TR. Nailfold capillary microscopy in healthy children and in childhood rheumatic diseases: A prospective single blind observational study. Ann Rheum Dis. 2003;62:444-9.
[Google Scholar]
|
| 7. |
Cony M, Klene-Boudard C, Fontan I, Sanciaume C, Sarrat P, Taieb A, et al. Periungual capillaroscopy patterns in normal children. Arch Fr Pediatr. 1992;49:171-4.
[Google Scholar]
|
| 8. |
Martino F, Agolini D, Aprigliano D, Guido F, Placanica G, Giardini O. Nailfold capillaroscopy in normal children between 0 and 16 years of age. Minerva Pediatr. 1997;49:197-201.
[Google Scholar]
|
| 9. |
Bhakuni DS, Vasdev V, Garg MK, Narayanan K, Jain R, Mullick G, et al. Nailfold capillaroscopy by digital microscope in an Indian population with systemic sclerosis. Int J Rheum Dis. 2012;15:95-101.
[Google Scholar]
|
| 10. |
Andrade LE, Gabriel Júnior A, Assad RL, Ferrari AJ, Atra E. Panoramic nailfold capillaroscopy: A new reading method and normal range. Semin Arthritis Rheum. 1990;20:21-31.
[Google Scholar]
|
| 11. |
Lee P, Leung FY, Alderdice C, Armstrong SK. Nailfold capillary microscopy in the connective tissue diseases: A semiquantitative assessment. J Rheumatol. 1983;10:930-8.
[Google Scholar]
|
| 12. |
Cutolo M, editor. Capillaroscopy in rheumatic diseases from the XVIII to the XXI century. In: Atlas of Capillaroscopy in Rheumatic Diseases. Milano: Elsevier Srl; 2010. p. 3-5.
[Google Scholar]
|
| 13. |
Cutolo M, Sulli A, Smith V. How to perform and interpret capillaroscopy. Best Pract Res Clin Rheumatol. 2013;27:237-48.
[Google Scholar]
|
| 14. |
Sebastiani M, Manfredi A, Vukatana G, Moscatelli S, Riato L, Bocci M, et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: A multicentre validation study. Ann Rheum Dis. 2012;71:67-70.
[Google Scholar]
|
| 15. |
De Angelis R, Grassi W, Cutolo M. A growing need for capillaroscopy in rheumatology. Arthritis Rheum 2009;61:405-10.
[Google Scholar]
|
| 16. |
Nagy Z, Czirják L. Nailfold digital capillaroscopy in 447 patients with connective tissue disease and Raynaud's disease. J Eur Acad Dermatol Venereol. 2004;18:62-8.
[Google Scholar]
|
| 17. |
Cutolo M, Grassi W, Matucci Cerinic M. Raynaud's phenomenon and the role of capillaroscopy. Arthritis Rheum. 2003;48:3023-30.
[Google Scholar]
|
| 18. |
Hoerth C, Kundi M, Katzenschlager R, Hirschl M. Qualitative and quantitative assessment of nailfold capillaries by capillaroscopy in healthy volunteers. Vasa. 2012;41:19-26.
[Google Scholar]
|
Fulltext Views
8,892
PDF downloads
3,902





