Translate this page into:
Skin biopsy–induced blistering in urticarial bullous pemphigoid
2 Delhi Dermpath Laboratory, 10, Aradhana Enclave, Ring Road, R K Puram, New Delhi, India
Correspondence Address:
Arshdeep
Delhi Dermatology Group, 10, Aradhna Enclave, Ring Road, RK Puram, New Delhi - 110 066
India
| How to cite this article: A, Batrani M, Kubba A, Kubba R. Skin biopsy–induced blistering in urticarial bullous pemphigoid. Indian J Dermatol Venereol Leprol 2020;86:552-555 |
Sir,
Bullous pemphigoid (BP) is the most common subepidermal immunobullous disorder, with protean cutaneous manifestations.[1] While the diagnosis of BP in the bullous stage is quite straightforward, it is often ambiguous in the urticarial stage or in atypical variants, as it mimicks spongiotic dermatitis or urticarial vasculitis for weeks or months. A rare subtype of trauma-induced BP represents an underreported entity in the literature.
A 61-year-old male presented to us with multiple red pruritic papules and nodules on chest, back, and lower limbs [Figure - 1]a for the last 2 years. ' He started developing annular plaques with erythematous, indurated coalescing margins along with central clearing and mild scaling, on trunk and limbs [Figure - 1]b since last 2 months. Few targetoid lesions were also noted on the ankles [Figure - 1]c. No history of drug intake (other than antihistamines) or associated systemic illness was elicited. A punch biopsy was performed from a representative lesion on right forearm with differential diagnosis of urticarial vasculitis, erythema multiforme, urticarial BP, and sarcoidosis. Interestingly, the patient returned after 48 hours, with multiple new blisters confined to the right forearm, around the biopsy site [Figure - 1]d. The bullae were tense, filled with serous fluid and variably sized. Remarkably, the bullae adjacent to the biopsy site were the largest. An additional biopsy (perilesional) was performed for direct immunofluorescence (DIF). Histopathology revealed mild acanthosis, subtle spongiosis, eosinophilic exocytosis and an upper dermal eosinophilic infiltrate distributed along the basal layer and forming an occasional microabscess in dermal papilla [Figure - 2]a, suggestive of urticarial stage of BP. DIF showed a strong linear band of C3 along the dermo–epidermal junction (DEJ) [Figure - 2]b, confirming the diagnosis of BP; however, immunoglobulins (Ig)G, IgM, and IgA were negative. The DIF specimen was split using 1 M normal saline to rule out epidermolysis bullosa acquisita. Routine blood biochemistry was normal except for the presence of peripheral eosinophilia (absolute eosinophil count 3,276/mm[3]) and nearly 15 times elevated serum IgE levels (2,572 IU/mL; normal range 10–200 IU/mL). The patient was started on potent topical steroids and subsequently he developed multiple new blisters over both preexisting urticarial plaques and normal skin after 1 month. The patient responded well to azathioprine (2 mg/kg), dapsone, and antihistamines and achieved remission within a year.
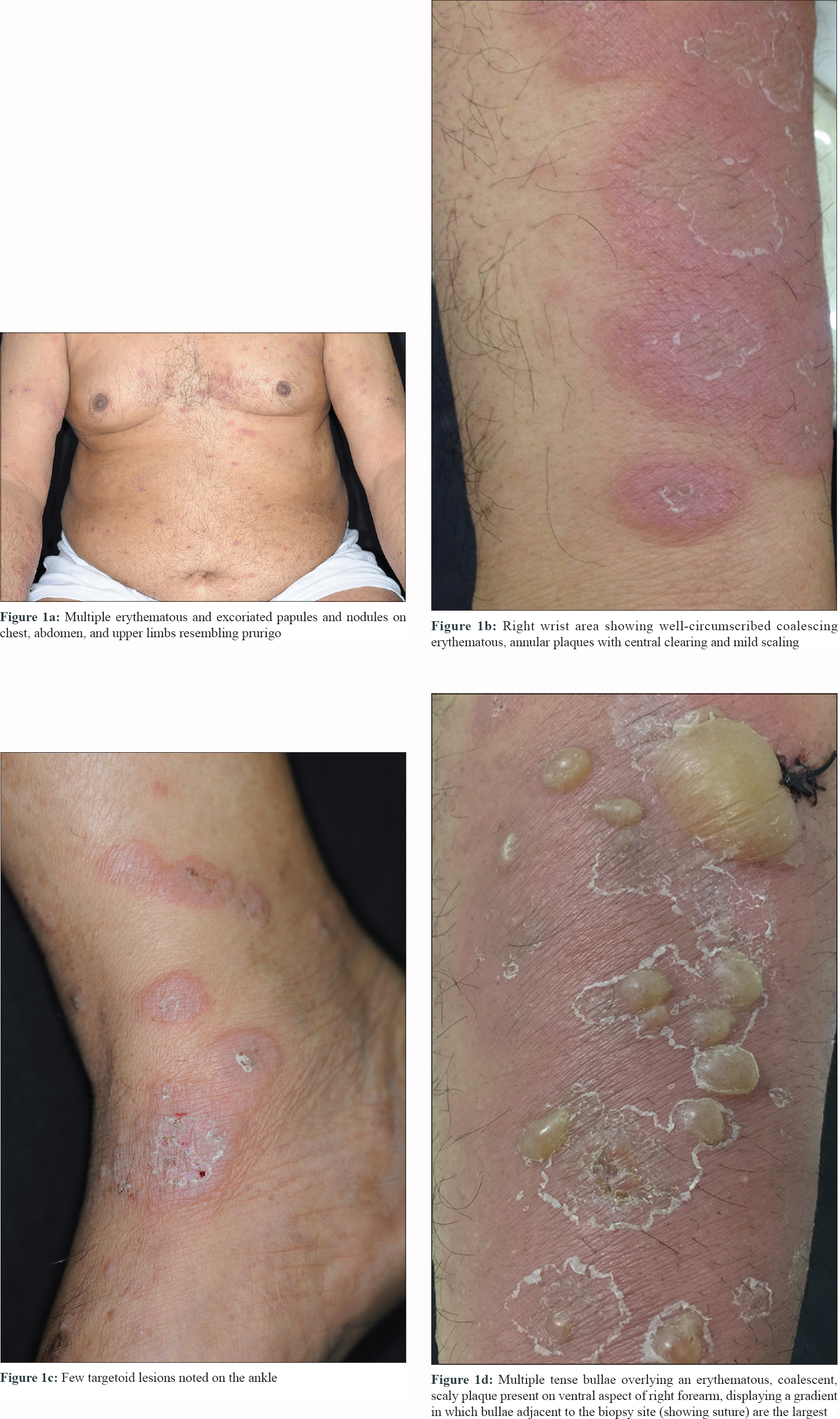 |
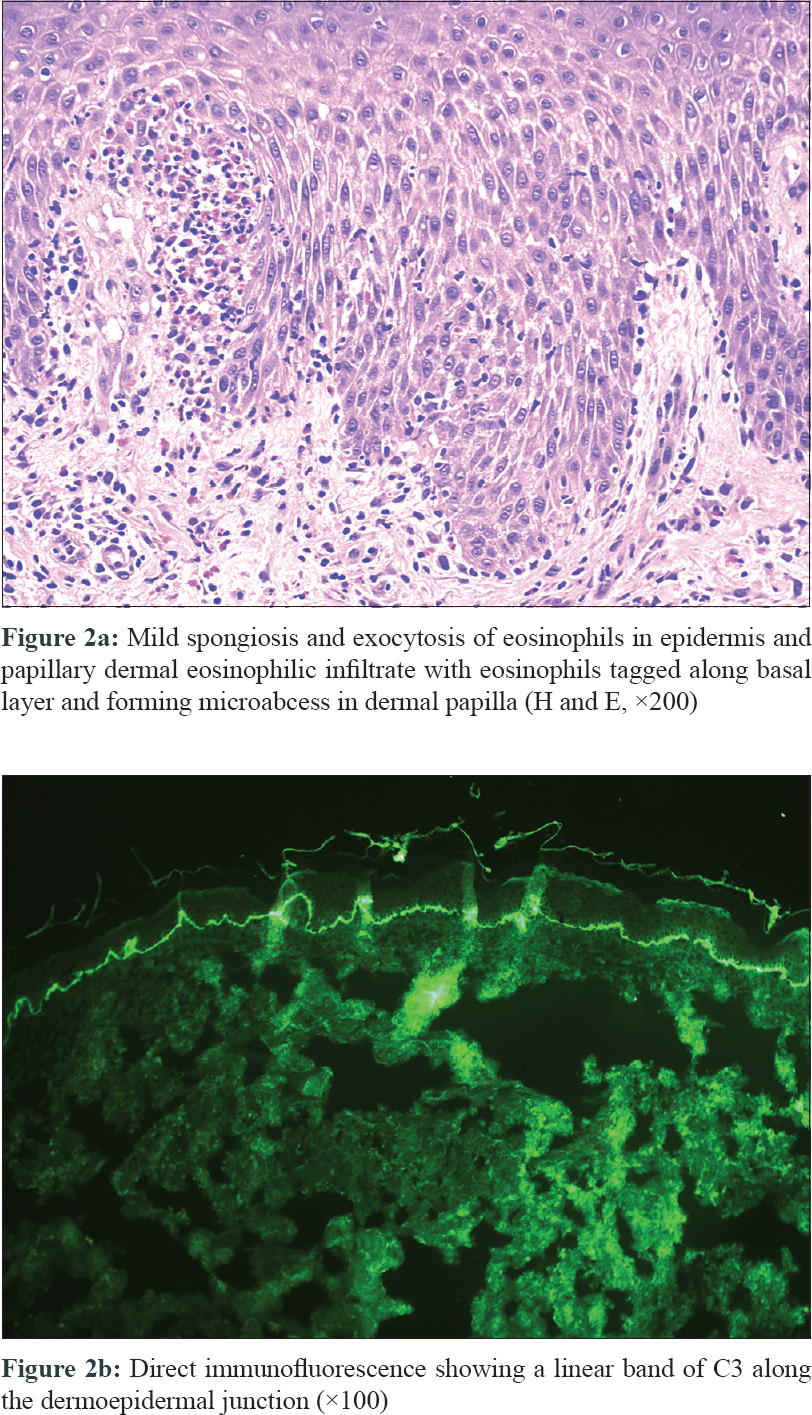 |
Herein, we have reported an unusual case of trauma-induced BP, where skin biopsy acted as the triggering factor. The characteristic display of decreasing gradient in size of bullae distal to the biopsy site (trauma) points toward trauma as an inciting factor. The biopsy procedure comprised of physical trauma induced by the punch and needle prick (for injecting local anesthetic) as well as the chemical stimuli of lignocaine and adrenaline (as local anaesthetic) and isopropyl alcohol swab (used for cleansing the skin). It is difficult to ascertain the exact triggering factor between the mechanical or chemical agents. Reports from literature suggest trauma as a trigger for the induction of BP, with occurrence of lesions at or around the site of burns,[2] irradiation,[3] surgery,[4] contrast dye injection,[5] insect bite,[5] topical medication (for psoriasis like tar, anthralin, and psoralens),[5] and also scabies.[6] In none of these reports, patients had a pre-existing blistering condition, hence this association cannot be called as a Koebner phenomenon by definition.[2] However, our casewith skin biopsy triggering the formation of bullae overlying preexisting urticarial plaques is a unique variant of trauma-induced BP.
A wide range of physical factors have been incriminated in the pathogenesis of this autoimmune blistering disorder. There is no consensus about the underlying mechanism involved, but several hypotheses have been proposed.[5] Animal studies in rabbit skin have shown that anti-BP230 autoantibodies can induce an enhanced inflammatory response only after an additional epithelial injury.[7] Dănescu et al. have proposed that low titers of autoantibodies are not pathogenic by themselves, as they do not induce the disease by simply binding to the antigen.[5] The subepidermal blistering mechanism includes activation of granulocytes and complement, which in turn induces activation of proteases and cytokines resulting in a cleft at the DEJ.[8] Trauma results in tissue destruction, triggering a cascade of proinflammatory stimuli and recruitment of inflammatory cells, particularly granulocytes. The autoantibodies binding to granulocytes further activate the complement and initiate the proteolytic enzymes to directly interfere with adhesion function of the autoantigens.[5],[8] Our case supports this hypothesis, as the patient presented with the urticarial stage of BP for 2 years, before trauma triggered the probable low titres of autoantibodies to precipitate the bullous stage of the disease.
Another hypothesis emphasizes the role of physical irradiation in altering the basement membrane and exposing the modified antigens, resulting in stimulation of autoantibodies and formation of antigen–antibody complexes, complement activation resulting in blister formation.[5] Literature supports a further hypothesis for radiation-induced BP that irradiation causes impaired immune reactivity of T-helper cells and T-suppressor cells, resulting in the development of antibodies targeted against the self-antigens.[9] Both these hypotheses are relevant for cases occurring around the sites of trauma like burns and irradiation, with induction of BP-specific autoantibodies per se. Topical medications like psoralens can also induce bullous lesions either directly by modulating the immune response or indirectly by causing local irritant reactions, which trigger a systemic immune response.[5] In our case, the role of chemical agents (isopropyl alcohol swab or injection lignocaine and adrenaline) in disease pathogenesis cannot be ruled out. In the index case, the time interval between trauma and onset of blistering on preexisting urticarial plaques was 2 days. In a review of nine patients with trauma-induced BP, five patients presented with specific lesions within few days of the trigger; the earliest being 1 h after trigger.[5] Seven patients (77.77%) presented with lesions progressing outside the afflicted area, with a tendency to become generalized, as also seen in our case.[5]
This phenomenon can be explained by the above-mentioned hypothesis that trauma causes activation of complement and subsequent inflammatory cascade, which may result in induction of blistering in areas of tissue-bound antibodies, even away from the site of trauma.
To conclude, physical factors pose an enigmatic trigger in the occurrence of BP, unlike other autoimmune blistering disorders. Further documentation and characterization of such cases are needed to unfold the underlying pathogenesis to help in treatment planning and search for newer therapeutic agents.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
De D, Khullar G, Handa S, Saikia UN, Radotra BD, Saikia B, et al. Clinical, demographic and immunopathological spectrum of subepidermal autoimmune bullous diseases at a tertiary center: A 1-year audit. Indian J Dermatol Venereol Leprol 2016;82:358.
[Google Scholar]
|
| 2. |
Korfitis C, Gregoriou S, Georgala S, Christofidou E, Danopoulou I. Trauma-induced bullous pemphigoid. Indian J Dermatol Venereol Leprol 2009;75:617-9.
[Google Scholar]
|
| 3. |
Sheerin N, Bourke JF, Holder J, North J, Burns DA. Bullous pemphigoid following radiotherapy. Clin Exp Dermatol 1995;20:80-2.
[Google Scholar]
|
| 4. |
Lo Schiavo A, Caccavale S, Alfano R, Gambardella A, Cozzi R. Bullous pemphigoid initially localized around the surgical wound of an arthroprothesis for coxarthrosis. Int J Dermatol 2014;53:e289-90.
[Google Scholar]
|
| 5. |
Dănescu S, Chiorean R, Macovei V, Sitaru C, Baican A. Role of physical factors in the pathogenesis of bullous pemphigoid: Case report series and a comprehensive review of the published work. J Dermatol 2016;43:134-40.
[Google Scholar]
|
| 6. |
Bornhövd E, Partscht K, Flaig MJ, Messer G. Bullous scabies and scabies-triggered bullous pemphigoid. Hautarzt 2001;52:56-61.
[Google Scholar]
|
| 7. |
Hall RP 3rd, Murray JC, McCord MM, Rico MJ, Streilein RD. Rabbits immunized with a peptide encoded for by the 230-kD bullous pemphigoid antigen cDNA develop an enhanced inflammatory response to UVB irradiation: A potential animal model for bullous pemphigoid. J Invest Dermatol 1993;101:9-14.
[Google Scholar]
|
| 8. |
Sesarman A, Oswald E, Chiriac MT, Csorba K, Vuta V, Feldrihan V, et al. Why human pemphigoid autoantibodies do not trigger disease by the passive transfer into mice? Immunol Lett 2012;143:92-100.
[Google Scholar]
|
| 9. |
Cliff S, Harland CC, Fallowfield ME, Mortimer PS. Localised bullous pemphigoid following radiotherapy. Acta Derm Venereol 1996;76:330-1.
[Google Scholar]
|
Fulltext Views
5,018
PDF downloads
1,749





