Translate this page into:
A randomized controlled trial of topical benzoyl peroxide 2.5% gel with a low glycemic load diet versus topical benzoyl peroxide 2.5% gel with a normal diet in acne (grades 1-3)
2 Department of Biochemistry, Kasturba Medical College Hospital, Manipal Academy of Higher Education, Mangalore, Karnataka, India
Correspondence Address:
Gatha M Upadya
Department of Dermatology, Kasturba Medical College Hospital, Attavar, Mangalore - 575 001, Karnataka
India
| How to cite this article: Pavithra G, Upadya GM, Rukmini M S. A randomized controlled trial of topical benzoyl peroxide 2.5% gel with a low glycemic load diet versus topical benzoyl peroxide 2.5% gel with a normal diet in acne (grades 1-3). Indian J Dermatol Venereol Leprol 2019;85:486-490 |
Abstract
Background: The improvement in insulin resistance and acne lesions on low glycemic load diets in various studies suggests that diet plays a significant role in acne pathogenesis.
Aims: To compare the efficacy of a low glycemic load diet plus topical benzoyl peroxide 2.5% gel with that of only topical benzoyl peroxide 2.5% gel in grades 1, 2 and 3 of acne vulgaris.
Methods: In a randomized controlled trial, 84 patients with grades 1, 2 and 3 acne vulgaris were divided into two groups, to receive a low glycemic load diet and no dietary intervention respectively. Acne lesions (face) were scored and graded at baseline and 4, 8 and 12 weeks. Homeostasis model assessment of insulin resistance and body mass index were measured during the first and last visits. Statistical analysis was done with Statistical Package for the Social Sciences, version 17.0.
Results: Both groups showed significant reduction in acne counts at 12 weeks (P = 0.931) with no statistically significant difference between the groups. The differences in body mass index and homeostasis model assessment of insulin resistance between the groups were statistically significant (P = 0.0001). Group 1 showed reductions in body mass index and homeostasis model assessment of insulin resistance values at the end of the study, whereas group 2 did not.
Limitations: Application of mild topical cleanser in both the groups might have contributed to the improvement in epidermal barrier function, and topical application of 2.5% of benzoyl peroxide gel in both groups contributed to the improvement in acne counts.
Conclusions: A low glycemic load diet did not result in any significant improvement in acne counts.
Introduction
Acne is a very common skin disorder affecting individuals of all age groups.[1],[2] Populations that consume low glycemic load diets, such as the Kitavan islanders of Guinea, Ache hunters, Inuits and rural Brazilians, tend to be acne-free.[3] An increase in the incidence of acne was observed in Inuits, Okinawa islanders and Chinese after they started following a western diet.[3] These observations suggest that lifestyle factors like diet may have a role in acne pathogenesis. A high glycemic load diet causes hyperinsulinemia, high androgen bioavailability, increased insulin-like growth factor-1 and acne.[4],[5] Increased insulin-like growth factor-1 further increases dihydrotestosterone and dehydroepiandrosterone sulfate, which increase sebum secretion and sebocyte proliferation.[4],[5] Increased insulin levels in blood elevate plasma levels of epidermal growth factor, transforming growth factor-β and free nonesterified fatty acids, thereby causing sebaceous gland inflammation and acne.[6] We conducted this study to determine the efficacy of a low glycemic load diet in the management of acne vulgaris.
Methods
The study was designed as a randomized controlled investigator-blinded trial. After obtaining institutional ethical committee clearance, a total of 84 patients (male and female, age 14–29 years) with acne of grades 1, 2 and 3 were included randomly from among dermatology outpatients in a tertiary care centre between September 2014 and May 2016. Patients already on topical medication, those on oral retinoid in the last 6 months or oral antibiotics in the last 15 days and pregnant women were excluded.
Patients were randomly assigned to two groups of 42 participants each, using the chit in the box method. Allotment was done by a registrar not involved in the rest of the study. Detailed history was taken from all participants including details of diet, duration of acne, treatment history, family history, personal history, cosmetic use and any specific aggravating factors.
Alow glycemic load diet (comprising 45% of energy from low glycemic load carbohydrates, 25% from proteins and 30% from fats) was advised for patients in Group 1.
(Glycemic load = Glycemic index of the food item × its carbohydrate content (g)/100. Glycemic index values were referred from a reference table,[7] from the Sydney University's Glycemic index website [8] and from the National Institute of Nutrition, ICMR, Hyderabad, India.[9])
Constituents of the low glycemic load diet were: Total calories: 1900 (±300 kcal) Carbohydrates: 47% (±5%). Proteins: 23% (±3%) Fats: 30% (±3%) [Table - 1].

Every participant in group 1 received an individualized diet plan from the dietitian on the first visit which matched their baseline diet. Group 1 patients maintained a diet chart with a daily menu which was examined during every visit to determine adherence.
Topical 2.5% benzoyl peroxide gel and a mild noncomedogenic cleanser were advised for all patients in both the groups.
Acne lesions (face) were scored and graded at all visits (baseline, 4, 8 and 12 weeks). Skin evaluation was done by modified Cunliffe–Leeds lesion count technique.[10] Body mass index and homeostasis model assessment of insulin resistance (fasting glucose mg/dl × fasting insulin μU/ml/405) were calculated at baseline and 12 weeks.
Statistical Package for the Social Sciences (SPSS) version 17.00 (SPSS Inc. 233, South Walker Drive, Chicago, IL) was used for statistical analysis. Student's unpaired t-test was used to analyze mean values of all quantitative variables. P < 0.05 was considered significant.
Sample size was determined by the formula: η = 2(Zα+ Zβ)[2]σ[2] / d [2]. With the power of study 90% and the confidence level of 95%, substituting in the above formula, the number of participants required for the study was 84. A total of 111 were enrolled in the study; though some patients were lost to follow-up and some more discontinued the treatment, we retained the required number [CONSORT Flowchart].

Results
The study included 42 individuals in group 1 who followed a low glycemic load diet and 42 patients in group 2 with no dietary intervention. Fifty-four (64.2%) of patients were in the age group of 20–24 years and a majority (57,67.8%) were women [Table - 2]. No significant differences were seen between the two groups in age distribution (P = 0.443) or sex distribution. Both groups had higher female populations (P = 0.815). A majority (68, 80.9%) in both the groups had grade 3 acne, with no significant difference in the grade of acne between the 2 groups (P = 0.581).
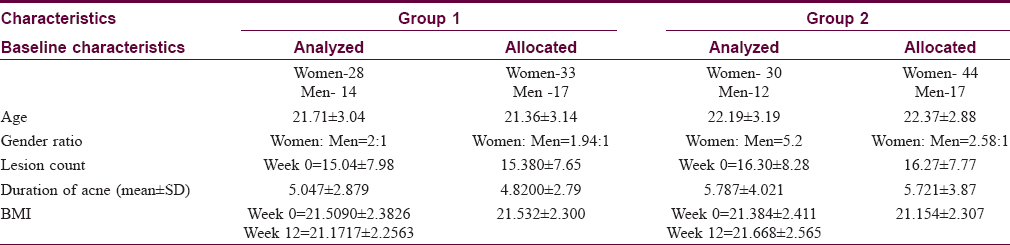
There was also no significant difference in the duration of acne between two groups. Duration of acne in most patients in our study was between 1 month and 5 years (P = 0.430). Thirty-eight (90.4%) patients in group 2 had a family history of acne, compared to18 (42.8%) patients in group 1 (P = 0.0001). Thirty one (73.8%) patients in group 2 had a history of usage of cosmetics compared to 28.5% of patients in group 1 (P = 0.0001).
Adherence to the low glycemic load diet in group 1 as determined from their daily diet chart was up to 75%.
Even though there was a significant reduction in acne counts at the end of the study in both the groups, [Figure - 1] and [Figure - 2] there was no significant difference [Table - 3] between the two groups (P = 0.931). The difference between group 1 and group 2 in respect to reduction of body mass index [Table - 4] during the study period was statistically significant (P = 0.0001). The difference in homeostasis model assessment of insulin resistance [Table - 5] during the study period between the two groups was also statistically significant (P = 0.0001).
 |
| Figure 1: Patient in Group 1 at 0 and 12 weeks |
 |
| Figure 2: Patient in Group 2 at 0 and 12 weeks |



Discussion
Nearly all individuals of 15-17 years age suffer from acne [11],[12],[13] and in 15–20% of young individuals, acne is moderate to severe.[11],[14],[15] Smith et al., in a randomized controlled trial on 43 male participants aged up to 25 years with mild to moderate facial acne, found that acne was a common problem faced by that age group.[16] The severity of acne vulgaris increases with maturity, and prepubertal girls with severe acne have higher dehydroepiandrosterone sulfate levels.[17],[18] In a German study, the authors found that 64% of people in the age group of 20-29 years and 43% of those in the age group of 30-39 years had acne.[19],[20]
A large Chinese study conducted among undergraduate students found that 78% of first-degree relatives had acne, which was heritable in nature.[21] Many retrospective studies have identified the genetic basis and clustering of familial cases in acne.[22],[23],[24],[25],[26]
In a cross-sectional study including Kitavan and Ache hunters, Cordain et al. observed that there was no acne in these populations consuming low glycemic load diets.[2] They proposed that a high glycemic load diet is a major factor contributing to acne in Western populations.[2] Kim et al. observed that consumption of fermented milk with lactoferrin decreases the total non-inflammatory and inflammatory lesion counts.[27] Kwon et al. also found that patients consuming low glycemic load diets showed decrease in the number of acne lesions after 5 weeks.[28] Di Landro et al. found that there was a positive association between acne and frequent consumption of milk and skimmed milk.[29] In our study, patients who consumed a low glycemic load diet showed decrease in body weight after a period of 12 weeks while those on a regular diet did not. Consuming high glycemic index and carbohydrate-rich foods repeatedly causes acute hyperinsulinemia in adolescents. Hyperinsulinemia has been implicated in acne pathogenesis due to its association with high a bioavailability of androgens and high concentrations of free insulin-like growth factor I.[4],[5] Rapid increase in insulin levels results in a rapid lowering of blood sugar. In turn, androgen released by the adrenal glands signals the liver to secrete glycogen from its storage to increase blood sugar towards normal. Decrease in blood sugar also stimulate food cravings. Finally, these cycles continue in a chronic manner. Frequent hyperinsulinemia increases appetite and then body weight and body mass index.[30]
Nagpal et al. conducted a cross-sectional study to determine the prevalence of insulin resistance and metabolic syndrome in men affected by acne and found that post-adolescent males with acne and higher body mass index more commonly have high insulin resistance.[31]
These studies indicate correlations between insulin resistance, body mass index and acne. In our study too, only group 1 patients had significant improvements in homeostasis model assessment of insulin resistance values, indicating that a low glycemic load diet helps decrease insulin resistance. However insulin resistance and body mass index values did not correlate with the number of acne lesions in our study .
Limitation of our study is application of mild topical cleanser in patients of both groups. Topical cleanser improves the normal skin barrier along with topical application of 2.5% of benzoyl peroxide gel in both groups. Topical benzoyl peroxide itself is a potent topical agent against acne. These might be the main factors responsible for significant reduction in acne counts in group 1 and group 2, so no significant difference in acne counts in between the two groups was observed. So a study on the effect of low glycemic load diet alone on acne may help us to overcome this limitation. Even the adherence to diet chart was only 75% in group 1 and this might be a limitation of the study. The other limitation would be that no specific diet chart was given to the group 2, so it is possible that some/many of them paid more attention to their diets once they were a part of the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Acknowledgement
We are grateful to Jestina Rachel Kurien for helping us to modify the manuscript.
Financial support and sponsorship
Partly sponsored by Manipal Academy of Higher Education.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Harper JC, Fulton J. Acne Vulgaris. Medicine; 15 July, 2008. Available from: http://www.emedicine.medscape.com/article/1069804-print. [Last accessed on 2008 Dec 15].
[Google Scholar]
|
| 2. |
Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J, et al. Acne vulgaris: A disease of Western civilization. Arch Dermatol 2002;138:1584-90.
[Google Scholar]
|
| 3. |
Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults. J Acad Nutr Diet 2014;114:384-92.
[Google Scholar]
|
| 4. |
Cordain L. Implications for the role of diet in acne. Semin Cutan Med Surg 2005;24:84-91.
[Google Scholar]
|
| 5. |
Cordain L, Eades MR, Eades MD. Hyperinsulinemic diseases of civilization: More than just syndrome X. Comp Biochem Physiol A MolIntegr Physiol 2003;136:95-112.
[Google Scholar]
|
| 6. |
Borisov N, Aksamitiene E, Kiyatkin A, Legewie S, Berkhout J, Maiwald T, et al. Systems-level interactions between insulin-EGF networks amplify mitogenic signaling. Mol Syst Biol 2009;5:256.
[Google Scholar]
|
| 7. |
Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5-6.
[Google Scholar]
|
| 8. |
Lindeberg S, Eliasson M, Lindahl B, Ahrén B. Low serum insulin in traditional pacific islanders – The Kitava study. Metabolism 1999;48:1216-9.
[Google Scholar]
|
| 9. |
Gopalan C, Rama Sastri, BV, Balasubramanian SC. National Institute of Nutrition (India) 1989, Nutritive value of Indian foods, Rev. ed./revised and updated by B.S. Narasinga Rao, YG. Deosthale and KC Pant, National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, India
[Google Scholar]
|
| 10. |
Burke BM, Cunliffe WJ. The assessment of acne vulgaris – The Leeds technique. Br J Dermatol 1984;111:83-92.
[Google Scholar]
|
| 11. |
Law MP, Chuh AA, Lee A, Molinari N. Acne prevalence and beyond: Acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clin Exp Dermatol 2010;35:16-21. [Erratum in: Clin Exp Dermatol 2010;35:339].
[Google Scholar]
|
| 12. |
Yahya H. Acne vulgaris in Nigerian adolescents – Prevalence, severity, beliefs, perceptions, and practices. Int J Dermatol 2009;48:498-505.
[Google Scholar]
|
| 13. |
Rademaker M, Garioch JJ, Simpson NB. Acne in schoolchildren: No longer a concern for dermatologists. BMJ 1989;298:1217-9.
[Google Scholar]
|
| 14. |
Lucky AW. A review of infantile and pediatric acne. Dermatology 1998;196:95-7.
[Google Scholar]
|
| 15. |
Wei B, Pang Y, Zhu H, Qu L, Xiao T, Wei HC, et al. The epidemiology of adolescent acne in North East China. J Eur Acad Dermatol Venereol 2010;24:953-7.
[Google Scholar]
|
| 16. |
Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: A randomized, investigator-masked, controlled trial. J Am Acad Dermatol 2007;57:247-56.
[Google Scholar]
|
| 17. |
Lucky AW, Biro FM, Huster GA, Morrison JA, Elder N. Acne vulgaris in early adolescent boys. Correlations with pubertal maturation and age. Arch Dermatol 1991;127:210-6.
[Google Scholar]
|
| 18. |
Lucky AW, Biro FM, Simbartl LA, Morrison JA, Sorg NW. Predictors of severity of acne vulgaris in young adolescent girls: Results of a five-year longitudinal study. J Pediatr 1997;130:30-9.
[Google Scholar]
|
| 19. |
Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: The risk of smoking. Br J Dermatol 2001;145:100-4.
[Google Scholar]
|
| 20. |
Cunliffe WJ, Gould DJ. Prevalence of facial acne vulgaris in late adolescence and in adults. BMJ Case Rep 1979;1:1109-10.
[Google Scholar]
|
| 21. |
Ballanger F, Baudry P, N'Guyen JM, Khammari A, Dréno B. Heredity: A prognostic factor for acne. Dermatology 2006;212:145-9.
[Google Scholar]
|
| 22. |
Bataille V, Snieder H, MacGregor AJ, Sasieni P, Spector TD. The influence of genetics and environmental factors in the pathogenesis of acne: A twin study of acne in women. J Invest Dermatol 2002;119:1317-22.
[Google Scholar]
|
| 23. |
Friedman GD. Twin studies of disease heritability based on medical records: Application to acne vulgaris. Acta Genet Med Gemellol (Roma) 1984;33:487-95.
[Google Scholar]
|
| 24. |
Walton S, Wyatt EH, Cunliffe WJ. Genetic control of sebum excretion and acne – A twin study. Br J Dermatol 1988;118:393-6.
[Google Scholar]
|
| 25. |
Sobral JF, Silva CNA, Rodrigues JC. Heritability and concordance of acne vulgarisemgemeos. Invest Clin Lab Ter 1997; 72: 417-20.
[Google Scholar]
|
| 26. |
Niermann H. Report on 230 twins with skin diseases. Z Mensch Vererb Konstitutionsl 1958;34:483-7.
[Google Scholar]
|
| 27. |
Kim J, Ko Y, Park YK, Kim NI, Ha WK, Cho Y, et al. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010;26:902-9.
[Google Scholar]
|
| 28. |
Kwon HH, Yoon JY, Hong JS, Jung JY, Park MS, Suh DH, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: A randomized, controlled trial. Acta Derm Venereol 2012;92:241-6.
[Google Scholar]
|
| 29. |
Di Landro A, Cazzaniga S, Parazzini F, Ingordo V, Cusano F, Atzori L, et al. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol 2012;67:1129-35.
[Google Scholar]
|
| 30. |
Berra B, Rizzo AM. Glycemic index, glycemic load: New evidence for a link with acne. J Am Coll Nutr 2009;28Suppl:450S-454S.
[Google Scholar]
|
| 31. |
Nagpal M, De D, Handa S, Pal A, Sachdeva N. Insulin resistance and metabolic syndrome in young men with acne. JAMA Dermatol 2016;152:399-404.
[Google Scholar]
|
Fulltext Views
7,437
PDF downloads
2,441





