Translate this page into:
Interleukin-17-dressed neutrophil: Neutrophil does not produce but delivers interleukin-17 to lesional epidermis causing keratinocyte S100A expression
Correspondence Address:
Keiichi Yamanaka
Department of Dermatology, Graduate School of Medicine, Mie University, 2-174 Edobashi, Tsu, Mie 514-8507
Japan
| How to cite this article: Mizutani K, Matsushima Y, Habe K, Yamanaka K. Interleukin-17-dressed neutrophil: Neutrophil does not produce but delivers interleukin-17 to lesional epidermis causing keratinocyte S100A expression. Indian J Dermatol Venereol Leprol 2019;85:531-534 |
Sir,
Because of the astonishing effect of anti-interleukin (IL)-17 antibody biologics on psoriasis, the importance of IL-17 is obvious in the clinical pathogenesis of psoriasis. IL-17 acts on keratinocytes to produce antimicrobial peptides, inflammatory proteins, and proinflammatory cytokines and chemokines including CXCL1 and CXCL8, which recruit neutrophils to the psoriatic lesions. Psoriatic lesions are characterized by epidermal proliferation, parakeratosis and neutrophil infiltration forming subcorneal microabscess. Until recently, neutrophils have been considered as IL-17 producing cells like Th17, γδ T cells, monocytes, NK cells, and type 3 innate lymphoid cells (ILC3) based on the presence of immunoreactive IL-17 on the neutrophils and IL-17 messenger RNA (mRNA) expression.[1] However, we have declared the absence of IL-17 mRNA expression in highly purified neutrophils, and IL-17 mRNAs are derived from contaminated lymphocytes and monocytes in patients with psoriasis.[2] These facts implicate the presence of IL-17-decorated neutrophil, “IL-17-dressed neutrophil,” in psoriasis as a transporter of IL-17 to the lesional epidermis.
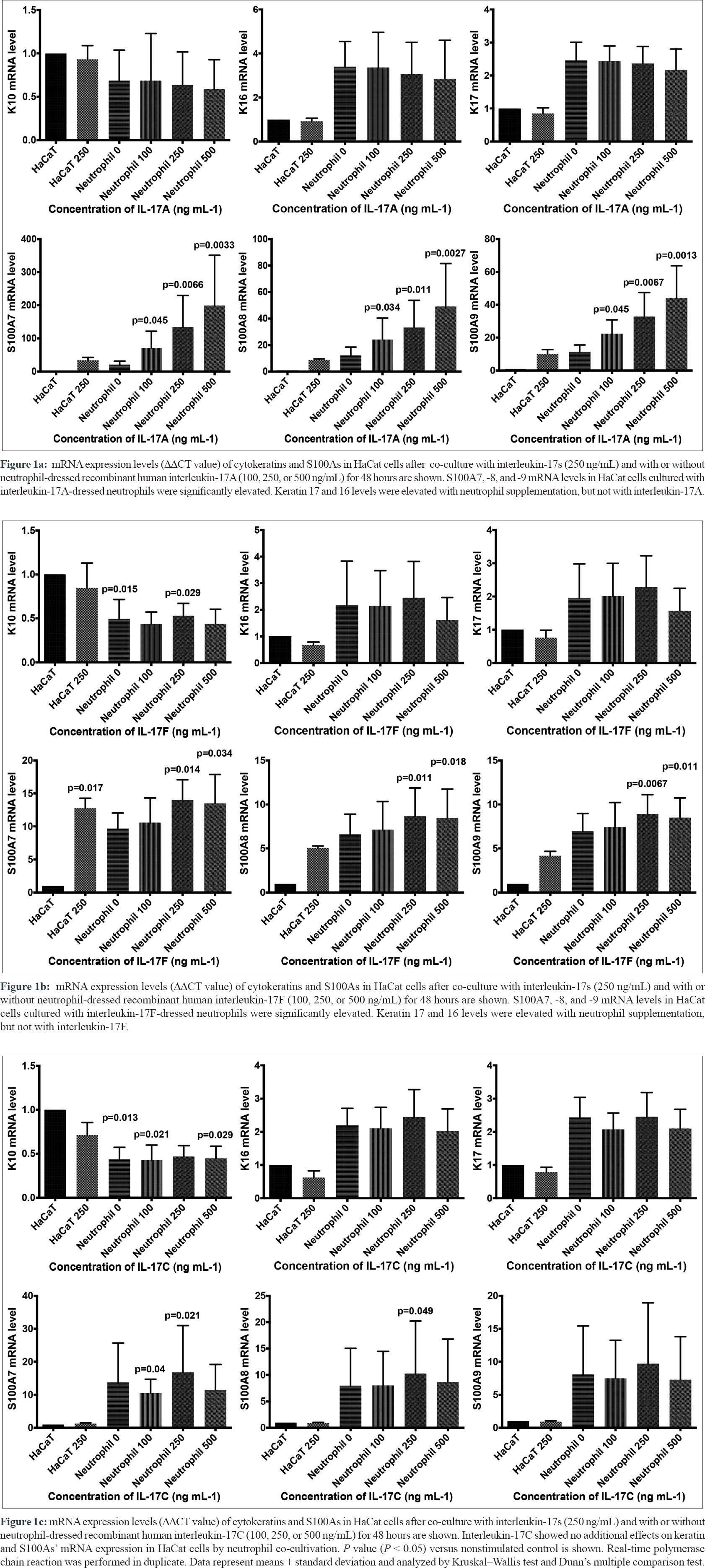
We here investigated the role of IL-17-dressed neutrophil in especially keratin (K) and S100A mRNA expression using a keratinocyte cell line, HaCat cells. HaCat cells (CLS Cell Lines Service, Eppelheim, Germany) were cultured in Dulbecco's Modified Eagle Medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Biosera, Ringmer, UK), 1% l-glutamine (LGL; Nacalai Tesque), and 1% penicillin–streptomycin (P/S; Sigma-Aldrich, St. Louis, MO, USA). The neutrophils were isolated by MACS ® Neutrophil Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) from the venous blood of the healthy volunteers (n = 4). The isolated neutrophils (1.0–3.0 × 10(6)/mL) were cultured in Roswell Park Memorial Institute (RPMI) 1640 (Nacalai Tesque) with 10% FBS, 1% LGL, and 1% P/S with or without recombinant human IL-17A (rhIL-17A; R and D Systems, Minneapolis, MN, USA), rhIL-17F (BioLegend, San Diego, CA, USA), or rhIL-17C (R and D Systems) for 2 hours. After washing twice, the neutrophils were cocultured with 40%–60% of confluent HaCat cells for 48 hours in DMEM/RPMI (1:1). Total RNA was extracted using TRI Reagent ® (Molecular Research Center, Cincinnati, OH, USA), and complementary DNA (cDNA) was synthesized using High-Capacity RNA-to-cDNA™ Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time polymerase chain reaction was performed using LightCycler ® 96 System (Roche Diagnostics, Mannheim, Germany) with TaqMan™ Universal Master Mix II, with UNG (Applied Biosystems). Primer sets K10, K16, K17, S100A7, S100A8, S100A9, and beta-actin (ACTB) for internal control were purchased from Applied Biosystems.
Upregulation of proliferation-related keratins, K16 and K17, and downregulation of K10 are characteristic in psoriatic epidermis.[3] In this study, HaCat cells treated by neutrophils either with or without IL-17A showed the elevation of K16 or K17 mRNA levels with marginal significance, and there was no obvious change in K10 mRNA expression [Figure - 1]a. This result suggests that the neutrophil itself activates keratinocytes, but IL-17A does not activate. Elevation of S100A7, -8, and -9 mRNA levels is also characteristic in psoriasis epidermis. Interestingly, S100A7, -8, and -9 mRNA levels in HaCat cells cultured with IL-17A-attached neutrophils were significantly elevated concentration dependently [Figure - 1]a. Effects of IL-17F-treated neutrophils on keratin mRNA expression of HaCat cells were similar to that of IL-17A [Figure - 1]b. S100A7, -8, and -9 mRNA levels were also elevated significantly with IL-17F-dressed neutrophils [Figure - 1]b. IL-17C showed no additional effects on keratin and S100As' mRNA expression in HaCat cells [Figure - 1]c.
 |
| Figure 1 |
Augmentation of K17 mRNA expression in the cultured keratinocytes by IL-17A has been reported previously; however, its expression was unchanged in HaCat cells in this study.[3] Neutrophil itself triggers the activation of keratinocyte. In contrast, S100A8 and -9 from keratinocytes have important role in neutrophil migration into the inflammatory lesions, and those expressions are significantly upregulated by IL-17A-decorated neutrophil dose dependently.[4] As we reported previously, neutrophils are not the major producers of IL-17A; however, neutrophils are possible transporters of IL-17A. This study declared that IL-17A adhered on to the neutrophils has no obvious effects on keratinocyte activation. However, IL-17A/F and neutrophils showed synergistic effects on S100As' expression in keratinocytes. This suggests that IL-17A/F-dressed neutrophils are functional and may carry IL-17s to the lesional epidermis and enhance neutrophil recruitment inducing keratinocytes S100As. K16 and K17 expressions were increased with the supplementation of neutrophils without IL-17, which suggests direct interaction of neutrophils, and keratinocytes promote keratinocyte activation without IL-17A. Interestingly, IL-17F- but not IL-17C-dressed neutrophils induced S100 expression as well as IL-17A, which suggests IL-17A/F but not IL-17C is a possible therapeutic target for psoriasis and IL-17-mediated diseases. However, the limitation is that we did not reveal the function of dressed neutrophil in vivo. The present results confirmed the biological roles of IL-17-dressed neutrophil and proposed a mechanism for anti-IL-17 therapies in psoriasis in part.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012;11:763-76.
[Google Scholar]
|
| 2. |
Yamanaka K, Yamagiwa A, Akeda T, Kondo M, Kakeda M, Habe K, et al. Neutrophils are not the dominant interleukin-17 producer in psoriasis. J Dermatol 2017;44:e170-1.
[Google Scholar]
|
| 3. |
Shi X, Jin L, Dang E, Chang T, Feng Z, Liu Y, et al. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J Invest Dermatol 2011;131:2401-8.
[Google Scholar]
|
| 4. |
Tardif MR, Chapeton-Montes JA, Posvandzic A, Pagé N, Gilbert C, Tessier PA. Secretion of S100A8, S100A9, and S100A12 by neutrophils involves reactive oxygen species and potassium efflux. J Immunol Res 2015;2015:296149.
[Google Scholar]
|
Fulltext Views
2,781
PDF downloads
1,721





