Translate this page into:
Complete response of a locally advanced basosquamous carcinoma with vismodegib treatment
2 Department of Pathology, University La Fe Hospital of Valencia Spain, Spain
3 Department of Oncology, University La Fe Hospital of Valencia Spain, Spain
4 Department of Dermatology, University La Fe Hospital of Valencia Spain; School of Medicine, University of Valencia, Spain
Correspondence Address:
Antonio Sahuquillo-Torralba
Department of Dermatology, University La Fe Hospital, Av. Fernando Abril Martorell 106, Valencia 46026
Spain
| How to cite this article: Sahuquillo-Torralba A, Llavador-Ros M, Caballero-Daroqui J, Botella-Estrada R. Complete response of a locally advanced basosquamous carcinoma with vismodegib treatment. Indian J Dermatol Venereol Leprol 2019;85:549-552 |
Sir,
Vismodegib is an inhibitor of the hedgehog pathway approved for the treatment of locally advanced and metastatic basal cell carcinoma. Complete responses were achieved in the pivotal ERIVANCE basal cell carcinoma study in 22.2% of the locally advanced treated tumors.[1] Nevertheless, its usefulness in tumors with mixed basaloid and squamous features (basosquamous) has not been clarified.
A 45-year-old woman, diagnosed with agoraphobia, consulted the emergency department for a large facial tumor, that according to the patient was present for 20 years and had been growing slowly. Physical examination showed a large ulcerated tumor, 13 cm in diameter, with well-defined margins that involved the forehead, glabella, dorsum of the nose and the inner aspect of the upper and lower eyelids on both sides. An overlying exophytic nodule was present [Figure - 1]a. No locoregional lymphadenopathy was detected and the neurological examination was normal. A computed tomography (CT) scan of the skull revealed an area of bone destruction measuring 4 × 5.5 cm, reaching the meningeal membranes and establishing contact with frontal lobes. There was no evidence of lymphatic or metastatic dissemination [Figure - 1]b.
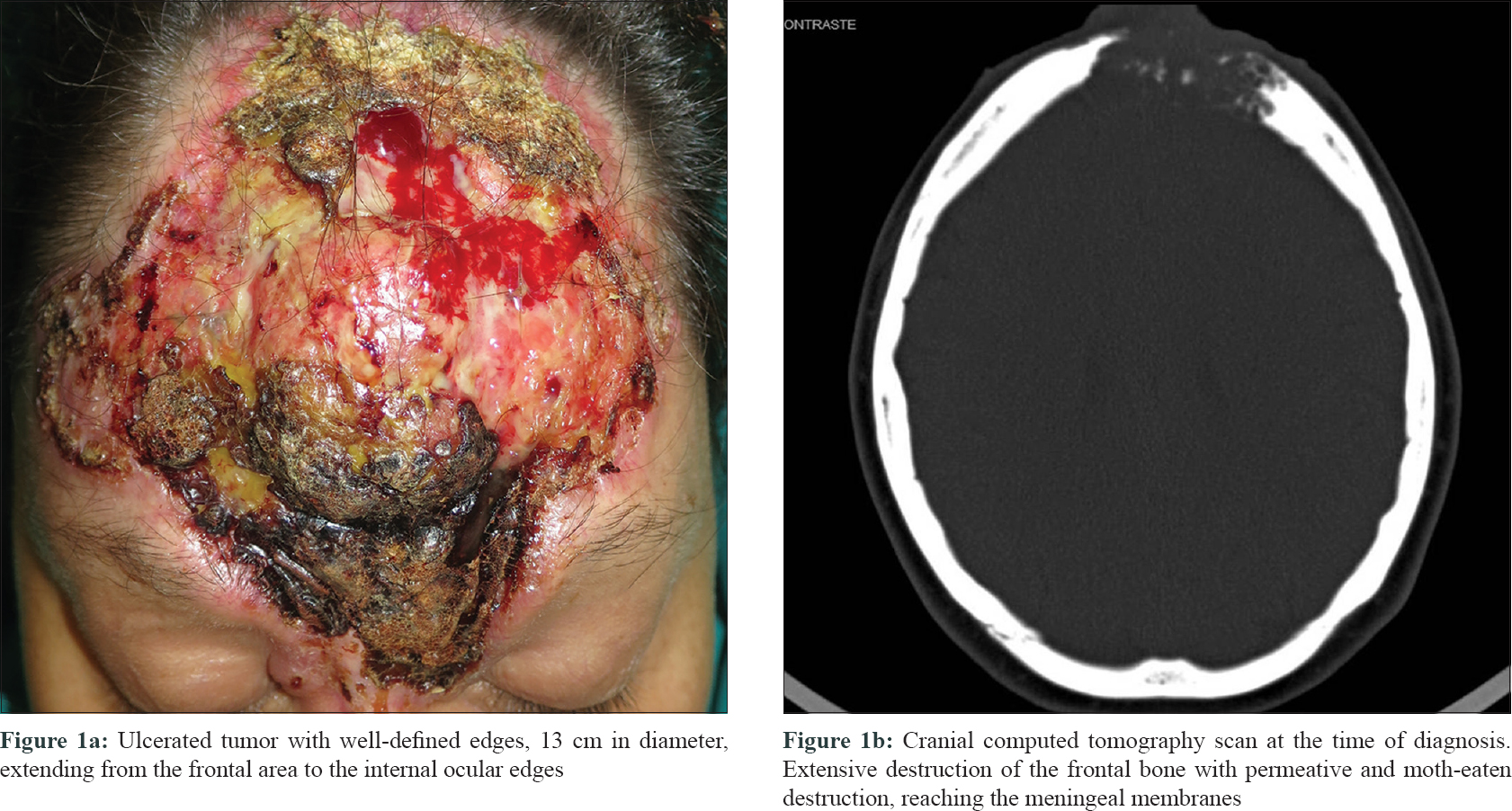 |
| Figure 1 |
Three biopsies were taken, two from the edges of the ulcer and one from the central nodule. All of them showed a proliferation of basaloid nests with palisading and stromal retraction that merged with areas of squamous differentiation [Figure - 2]a and [Figure - 2]b. Immunolabeling with Ber-Ep4 highlighted the basaloid cells, but was negative in the areas with squamous differentiation [Figure - 2]c, while cytokeratin AE1/AE3 (CK AE1/AE3) was diffusely positive in the squamotized areas [Figure - 2]d, with positivity of CAM-5 confirming the diagnosis of basosquamous carcinoma. Surgical treatment was not considered feasible and vismodegib treatment was initiated at an approved dose of 150 mg/day. Three months later, a remarkable reduction in tumor size was documented with complete response after 7 months, following which the treatment was stopped [Figure - 3]a. CT skull performed at this time showed bone remineralization and fibrotic changes that suggested absence of tumor [Figure - 3]b. Adverse events included progressive alopecia, dysgeusia and muscle spasms that started in the fourth month and were progressive since the treatment was stopped. Nine months after treatment withdrawal, no recurrence was detected.
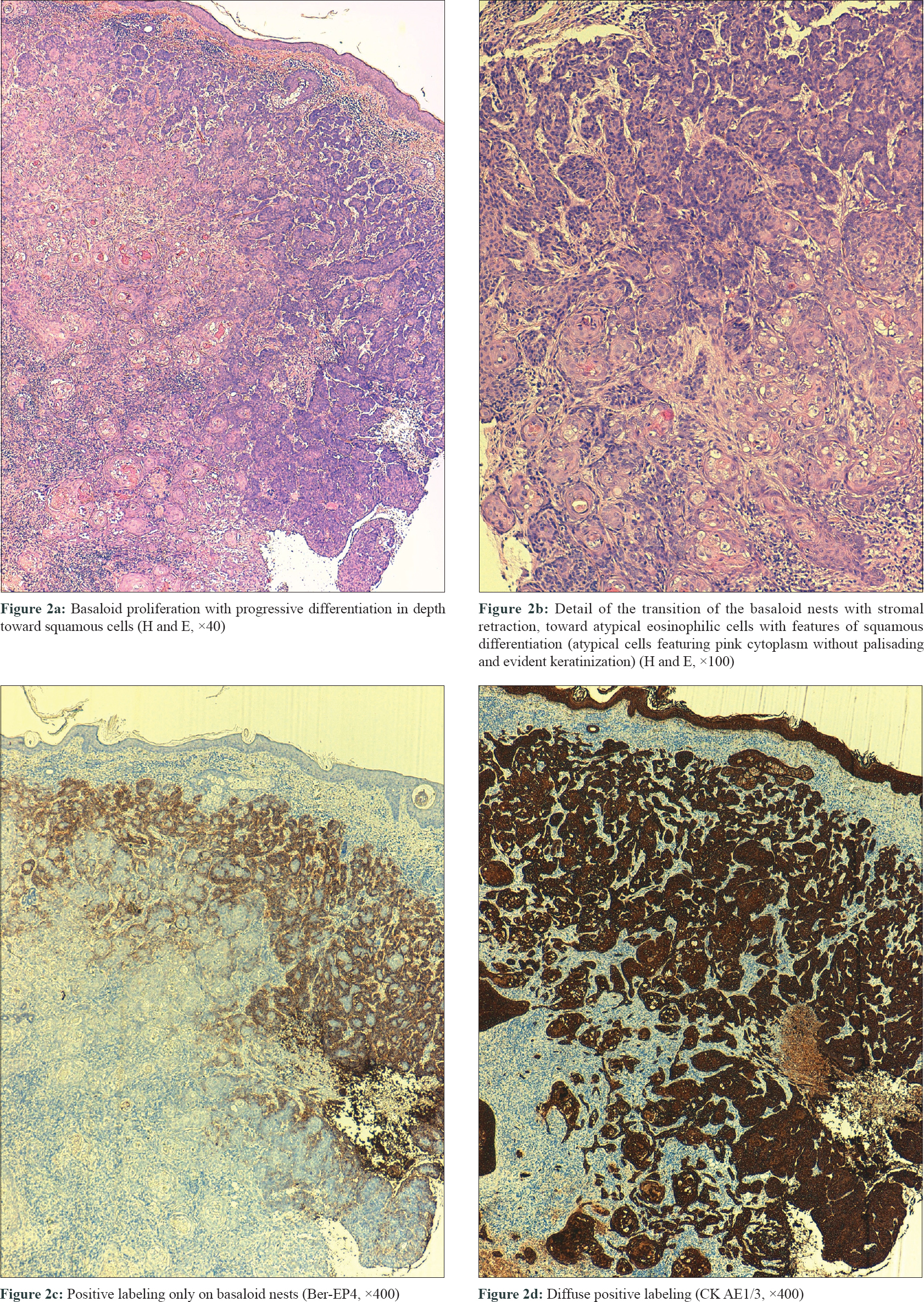 |
| Figure 2 |
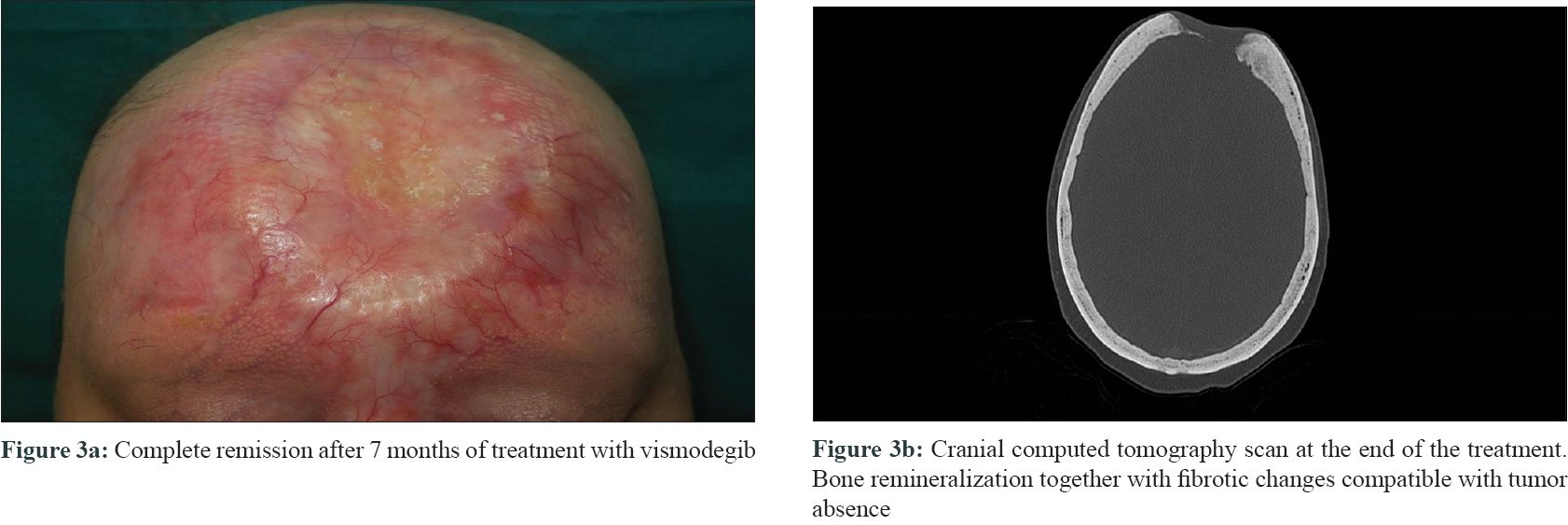 |
| Figure 3 |
Basosquamous carcinoma is an uncommon variant of basal cell carcinoma, characterized by a basaloid neoplastic proliferation with foci of squamous differentiation indistinguishable from those present in squamous cell carcinoma. The main differential diagnosis of basosquamous/metatypical carcinomas is the squamous cell carcinoma, which occasionally may have areas of basaloid differentiation. Immunohistochemical staining can be useful in confirming the diagnosis, since basal cell carcinomas are Ber-EP4-positive and squamous cell carcinomas are always negative for this staining. In basosquamous tumors, a gradation in this labeling has been found as the tumor moves from the basaloid to the squamous areas.[2] Due to the squamous component, basosquamous carcinoma carries a high risk of recurrence and metastasis.
Treatment of basosquamous carcinoma does not differ from the rest of the basal cell carcinomas and surgery is considered the standard of care. Nevertheless, when it becomes locally advanced or metastatic, the treatment may not remain amenable to surgery or radiation therapy and blocking the oncogenic sonic hedgehog pathway becomes necessary. Vismodegib is the first Food and Drug Administration–approved drug for this purpose, blocking 'smoothened', a transmembrane protein of the sonic hedgehog pathway. Other known inhibitors for this pathway are sonidegib: With the same mechanism of action like vismodegib and similar efficacy results, and itraconazole. The latter acts on the essential Hh pathway component Smoothened (SMO) in a distinctive way from the other inhibitors and with less efficacy demonstrated.[3] Administration of vismodegib to basosquamous carcinomas requires some considerations, since the appearance of squamous cell carcinoma in the original basal cell carcinoma tumor bed has been reported during vismodegib therapy. This phenotype switching is infrequent and does not preclude its use in basosquamous carcinomas.
We found only one case of basosquamous carcinoma treated with vismodegib reported in the literature; however, the drug had to be withdrawn due to adverse effects before evaluation of its effectiveness could be done. In that case, the patient was eventually treated with nivolumab, achieving a stable disease situation.[4] This response of basosquamous carcinoma to immunotherapy is not an isolated event, since we found other published cases of classic forms of locally advanced or metastatic basal cell carcinoma, as well as squamous cell carcinoma being treated with immunotherapy with good response.[5] In fact, there is currently one phase II clinical trial underway (clinicaltrials.gov), evaluating the role of pembrolizumab in locally advanced or metastatic basal cell carcinoma after progression or partial response to vismodegib (NCTO2690948).
In conclusion, we present the complete regression of a locally giant advanced basosquamous carcinoma with bone involvement, after only 7 months of treatment with vismodegib. We were unable to find any previous similar reports published to date.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Sekulic A, Migden MR, Lewis K, Hainsworth JD, Solomon JA, Yoo S, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol 2015;72:1021-6.e8.
[Google Scholar]
|
| 2. |
Jones MS, Helm KF, Maloney ME. The immunohistochemical characteristics of the basosquamous cell carcinoma. Dermatol Surg 1997;23:181-4.
[Google Scholar]
|
| 3. |
Wahid M, Jawed A, Mandal RK, Dar SA, Khan S, Akhter N, et al. Vismodegib, itraconazole and sonidegib as hedgehog pathway inhibitors and their relative competencies in the treatment of basal cell carcinomas. Crit Rev Oncol Hematol 2016;98:235-41.
[Google Scholar]
|
| 4. |
Borradori L, Sutton B, Shayesteh P, Daniels GA. Rescue therapy with anti-programmed cell death protein 1 inhibitors of advanced cutaneous squamous cell carcinoma and basosquamous carcinoma: Preliminary experience in five cases. Br J Dermatol 2016;175:1382-6.
[Google Scholar]
|
| 5. |
Winkler JK, Schneiderbauer R, Bender C, Sedlaczek O, Fröhling S, Penzel R, et al. Anti-programmed cell death-1 therapy in nonmelanoma skin cancer. Br J Dermatol 2017;176:498-502.
[Google Scholar]
|
Fulltext Views
3,286
PDF downloads
1,755





