Translate this page into:
Mycobacterium chelonae infection complicating traumatic and surgical wounds: A case series
2 Department of Microbiology, Amrita Institute of Medical Sciences, Kochi, Kerala, India
Correspondence Address:
Soumya Jagadeesan
Department of Dermatology, Amrita Institute of Medical Sciences, Kochi, Kerala
India
| How to cite this article: Jagadeesan S, Anilkumar V, Panicker VV, Anjaneyan G, Thomas J. Mycobacterium chelonae infection complicating traumatic and surgical wounds: A case series. Indian J Dermatol Venereol Leprol 2018;84:45-48 |
Abstract
Mycobacterium chelonae is a rapidly growing non-tuberculous mycobacterium. The skin and soft tissue infections due to this organism are steadily on the rise and need to be delineated specifically as most of these are not responsive to routine antituberculosis treatment. Here, we report 3 different presentations caused by Mycobacterium chelonae in traumatic and surgical wounds. Mycobacterium chelonae can complicate surgical or traumatic wounds.This infection may also present as injection site abscesses. Diabetics on insulin injections are especially at risk. A high index of suspicion is necessary in long standing culture negative lesions for clinching the diagnosis. PCR can be helpful in confirming the diagnosis.Introduction
Mycobacterium chelonae is a rapidly growing non-tuberculous mycobacterium, classified as a Runyon Group IV mycobacterium. Although ubiquitous in the environment, it can be a rare cause of infection in humans, especially in surgical and traumatic wounds. The organisms are resistant to conventional antituberculous agents and have varied susceptibilities to other antimycobacterial agents. Here, we report three different presentations caused by M. chelonae in traumatic and surgical wounds.
Case Reports
Case 1
A 72-year-old woman who was a social worker presented to the dermatology department with multiple painful swellings on the abdomen with purulent discharge of 10 weeks duration. At the time of presentation, she was being treated with various broad-spectrum antibiotics and had undergone surgical drainage many times, despite which the lesions failed to heal. She denied a history of trauma or surgical procedures but conceded taking insulin injections at the same sites, without aseptic precautions. Gram staining of smears and cultures were repeated which revealed no growth. A possibility of atypical mycobacterial infection was considered. Ziehl–Neelsen staining of smears showed acid-fast bacilli and mycobacterial cultures grew nonpigmented colonies on the 6th day. Isolate was presumptively identified as M. chelonae and was confirmed using multiplex polymerase chain reaction (PCR) (Mycobacterium Chip, XCyton Diagnostics Pvt. Ltd., Bengaluru, Karnataka, India). Treatment with doxycycline 100 mg and clarithromycin 500 mg twice daily was started. After 2 weeks, the patient developed severe diarrheal episodes, following which clarithromycin was substituted with linezolid 600 mg twice daily. The lesions healed fully within 4 weeks. Treatment was continued for a total duration of 10 weeks with no recurrence during the 6 months follow-up period.
Case 2
A 68-year-old man who was a retired pharmacist presented with a painful verrucous ulcerated plaque on the left second toe [Figure 1a] for past 4 months. He recalled a minor traumatic abrasion on the same site with a vegetative matter before the onset of this lesion. He was treated with several courses of broad-spectrum antibiotics which did not show any response. Skin biopsy revealed intradermal abscess formation with neutrophilic infiltration. Mantoux was negative; chest X-ray showed no evidence of tuberculosis. Mycobacterial culture showed rapidly growing nonpigmented colonies of acid-fast bacteria and the causative organism was confirmed as M. chelonae by multiplex PCR. He was treated with clarithromycin and doxycycline and the ulcer healed in 6 weeks [Figure 1b]. The treatment was continued for 4 more weeks after the resolution of the lesion and there was no recurrence during a follow-up period of 3 months.
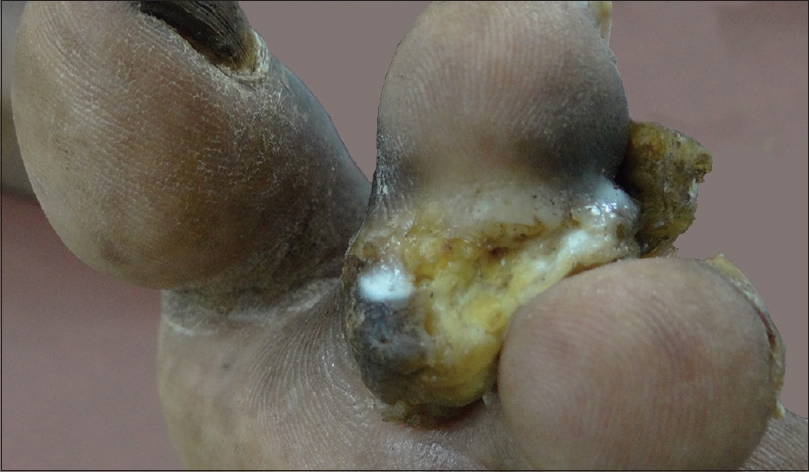 |
| Figure 1a: Verrucous ulcerated plaque on the toe in case 2 - At presentation |
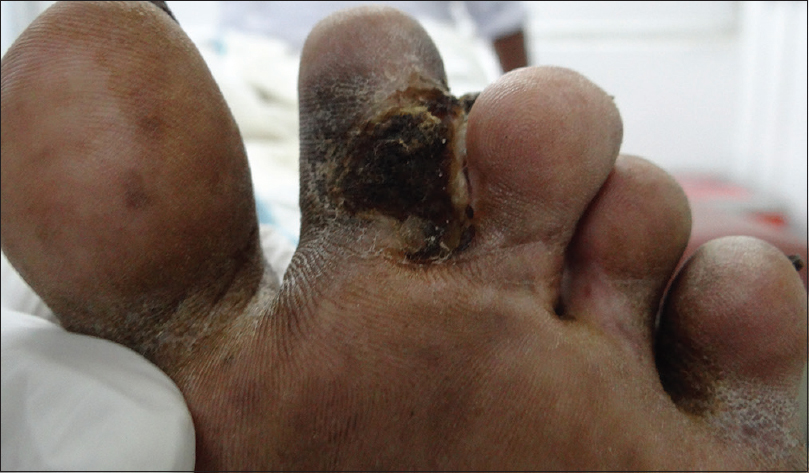 |
| Figure 1b: After 6 weeks of treatment |
Case 3
A 36-year-old lady who was a homemaker presented with a 6-month history of recurrent reddish raised lesions on the abdomen which were extremely tender. She had undergone a laparoscopic cystectomy for chocolate cyst (endometrioma), 2 months following which she noticed the lesions, initially on the laparoscopic port sites with persistent discharge. She was treated with multiple antibiotics despite which she developed new lesions on the adjoining skin. As a previous biopsy had revealed granulomatous inflammation, she was taking antituberculous treatment for the past 2 months from a local hospital. A cutaneous examination revealed multiple erythematous nodules and sinuses discharging pus on the anterior abdominal wall [Figure 2a]. Microscopy, bacterial and mycobacterial cultures were noncontributory. However, tissue samples were subjected to multiplex PCR which detected M. chelonae. Antituberculous treatment was stopped (which was showing only minimal response even after 11 weeks) and the patient was prescribed clarithromycin 500 mg and ofloxacin 200 mg tablets twice daily. Ofloxacin was considered in this case because the patient had been previously treated with doxycycline from another center before starting antituberculous treatment with minimal response. She was reviewed on a weekly basis and there was a good response within 3 weeks. All the lesions healed completely in 6 weeks [Figure 2b]. The treatment was stopped after 12 weeks with no recurrence in a follow-up period of 3 months.
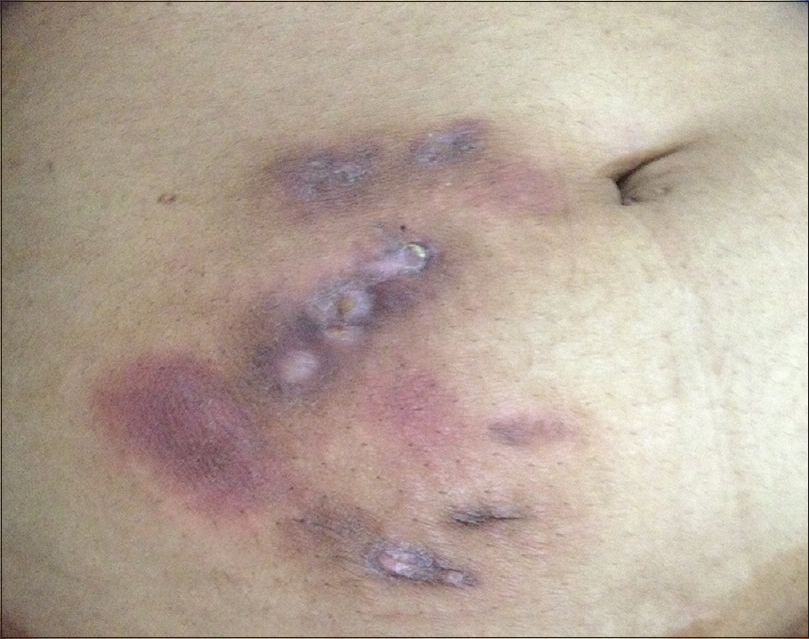 |
| Figure 2a: Erythematous nodules with discharging sinuses on the right anterior abdominal wall - At presentation |
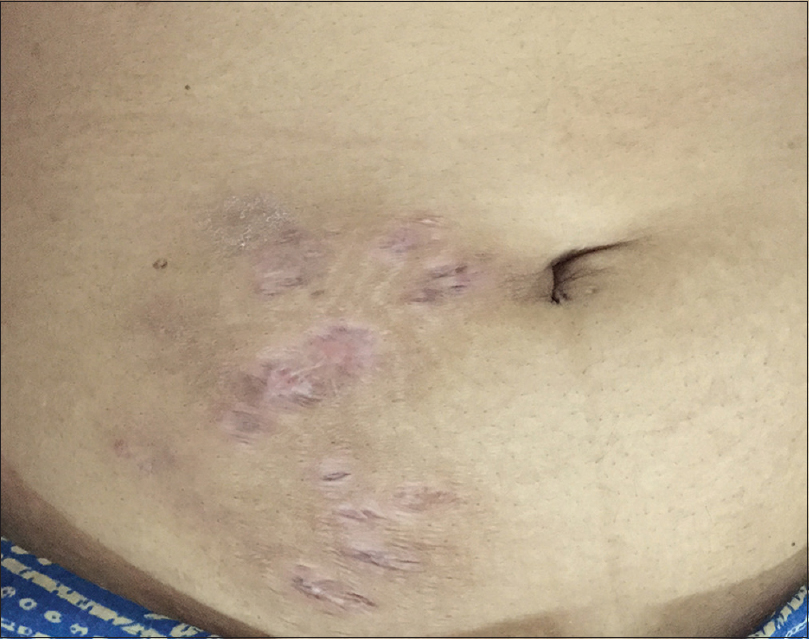 |
| Figure 2b: After 6 weeks of treatment |
Discussion
M. chelonae is named after the sea turtle, Chelona corticata, from which it was first isolated. Infections due to this environmental pathogen are on the rise and there have been reports of skin and soft tissue infections, infections involving skeletal tissue, eye, ear, lung and rare cases of endocarditis and meningitis. Most of these infections except lung disease occur following trauma.[1]
Cutaneous infections manifest as localized cellulitis, granulomatous nodules, sinuses, abscesses and ulcers. Primary cutaneous infections have been reported after insulin injections in diabetics.[2],[3],[4] In the first case, the portal of entry was suspected to be the site of insulin injections; the woman was using a pen injector on the abdomen. There are reports of nonhealing leg ulcers occurring due to M. chelonae, occurring after penetrating injuries which may have been the route of infection in the second case.[5]
M. chelonae-associated surgical site and wound infections are frequently associated with contaminated tap water. A hospital outbreak in India was associated with water used to rinse endoscopes for laparoscopic surgery, resulting in 145 wound infections in 35 patients.[6],[7] In the third case, the route of infection was probably through the use of contaminated laparoscope. Sterilization of laparoscopes for 30 min in 2% alkaline glutaraldehyde solution is recommended to avoid such infections. Skin and soft tissue infections have also been reported following acupuncture, tattooing, mesotherapy and other cosmetic procedures.[8]
Isolation of the organism is best accomplished by culture at 28–30°C which usually grows within 7 days of inoculation [Figure 3a] and [Figure 3b]. Once colonies are isolated, biochemical tests can be performed for identification of species. Molecular identification methods are useful for differentiating among the rapid growers and give rapid and accurate results.[8] In our cases, DNA amplification was done using multiplex PCR which could simultaneously detect Mycobacterium fortuitum, M. chelonae and Mycobacterium tuberculosis (XCyton Diagnostics Pvt. Ltd., Bengaluru, Karnataka, India).
 |
| Figure 3a: Nonpigmented colonies of Mycobacterium chelonae in culture |
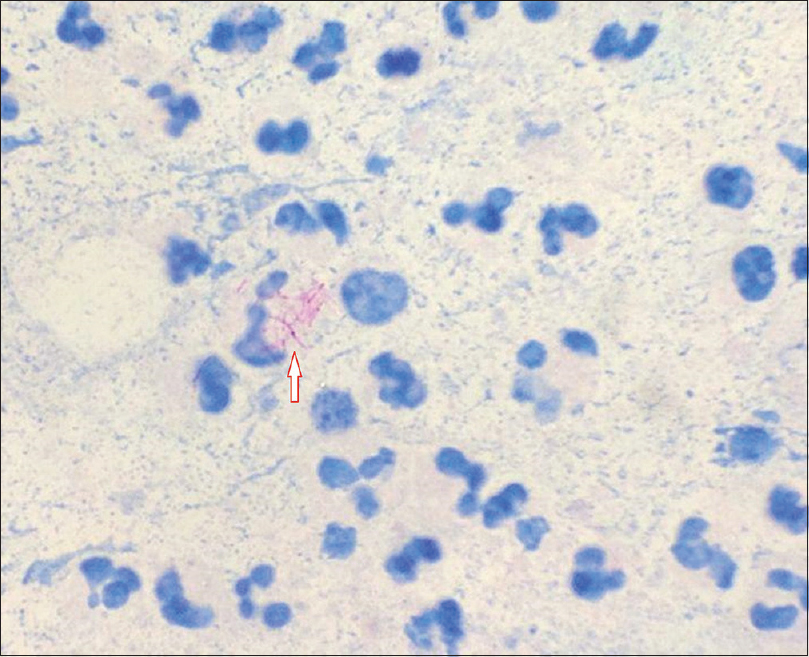 |
| Figure 3b: Smear showing the acid-fast rods |
Macrolide agents are the cornerstone of medical therapy for M. chelonae infections and generally, clarithromycin is the preferred agent. Aminoglycosides also show excellent activity against M. chelonae. Fluoroquinolones and tetracyclines too are effective agents and there are recent reports of excellent sensitivity to linezolid.[9] Regardless, monotherapy should be undertaken with caution; development of resistance to individual agents has been reported.
Conclusion
We wish to highlight the various presentations and modes of infection of M. chelonae in skin. A high index of suspicion is necessary for clinching the diagnosis, especially in culture-negative cases. In all our cases, there was a minimum lag of 3 months from the onset to diagnosis which could have been avoided. PCR-based tests may help in rapid and accurate diagnosis in suspected cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Mankad S, Karthik R, Rupali P, Michael JS. Fatal disseminated Mycobacterium chelonae infection in an immunocompromised host – A unique presentation. J Assoc Physicians India 2015;63:49-52.
[Google Scholar]
|
| 2. |
Jackson PG, Keen H, Noble CJ, Simmons NA. Injection abscesses in a diabetic due to Mycobacterium chelonei var abscessus. BMJ 1980;281:1105-6.
[Google Scholar]
|
| 3. |
Kelly SE. Multiple injection abscesses in a diabetic caused by Mycobacterium chelonei. Clin Exp Dermatol 1987;12:48-9.
[Google Scholar]
|
| 4. |
Finucane K, Ambrey P, Narayan S, Archer CB, Dayan C. Insulin injection abscesses caused by Mycobacterium chelonae. Diabetes Care 2003;26:2483-4.
[Google Scholar]
|
| 5. |
Terry S, Timothy NH, Zurlo JJ, Manders EK. Mycobacterium chelonae: Nonhealing leg ulcers treated successfully with an oral antibiotic. J Am Board Fam Pract 2001;14:457-61.
[Google Scholar]
|
| 6. |
Vijayaraghavan R, Chandrashekhar R, Sujatha Y, Belagavi CS. Hospital outbreak of atypical mycobacterial infection of port sites after laparoscopic surgery. J Hosp Infect 2006;64:344-7.
[Google Scholar]
|
| 7. |
Rajini M, Prasad SR, Reddy RR, Bhat RV, Vimala KR. Postoperative infection of laparoscopic surgery wound due to Mycobacterium chelonae. Indian J Med Microbiol 2007;25:163-5.
[Google Scholar]
|
| 8. |
Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med 2014;138:1106-9.
[Google Scholar]
|
| 9. |
Brown-Elliott BA, Wallace RJ Jr., Blinkhorn R, Crist CJ, Mann LB. Successful treatment of disseminated Mycobacterium chelonae infection with linezolid. Clin Infect Dis 2001;33:1433-4.
[Google Scholar]
|
Fulltext Views
6,955
PDF downloads
2,286





