Translate this page into:
Leukemia cutis mimicking erythema nodosum or vice versa: A histological conundrum
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
3 Department of Hematology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Sunil Dogra
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Chandigarh
India
| How to cite this article: Vinay K, Chatterjee D, Yanamandra U, Saikia UN, Malhotra P, Dogra S. Leukemia cutis mimicking erythema nodosum or vice versa: A histological conundrum. Indian J Dermatol Venereol Leprol 2018;84:91-93 |
Sir,
Leukemia cutis is a specific cutaneous manifestation of lymphoproliferative disorders, characterized by localized or disseminated infiltration of the skin by malignant leukemic cells.[1] Furthermore, circulating leukemic cells are known to infiltrate sites of trauma, burns, herpes simplex/herpes zoster, and skin tumors; this represents spillover of circulating blast cells into areas of inflammation.[2] Here we report an interesting case of leukemic infiltrates causing septal panniculitis, which clinically resembled erythema nodosum.
A 31-year-old male patient, diagnosed to have chronic myeloid leukemia in accelerated phase, presented to the Postgraduate Institute of Medical Education and Research, Chandigarh for evaluation of acute-onset tender nodular lesions over his shins, since the past 5 days. One week prior to the onset of these symptoms, he had suffered from a respiratory tract infection, which was treated with oral antibiotics by a primary care physician. His current medications included imatinib mesylate 800 mg/day and folic acid. The patient was febrile at presentation with a body temperature of 101°F. On mucocutaneous examination, symmetrically distributed, multiple, tender, nodular lesions of varying size were noted over the extensor aspects of lower limbs [Figure - 1]. There was no evidence of ulceration in any of the skin lesions. Clinical possibilities of erythema nodosum, Sweet's syndrome, and polyarteritis nodosa were considered.
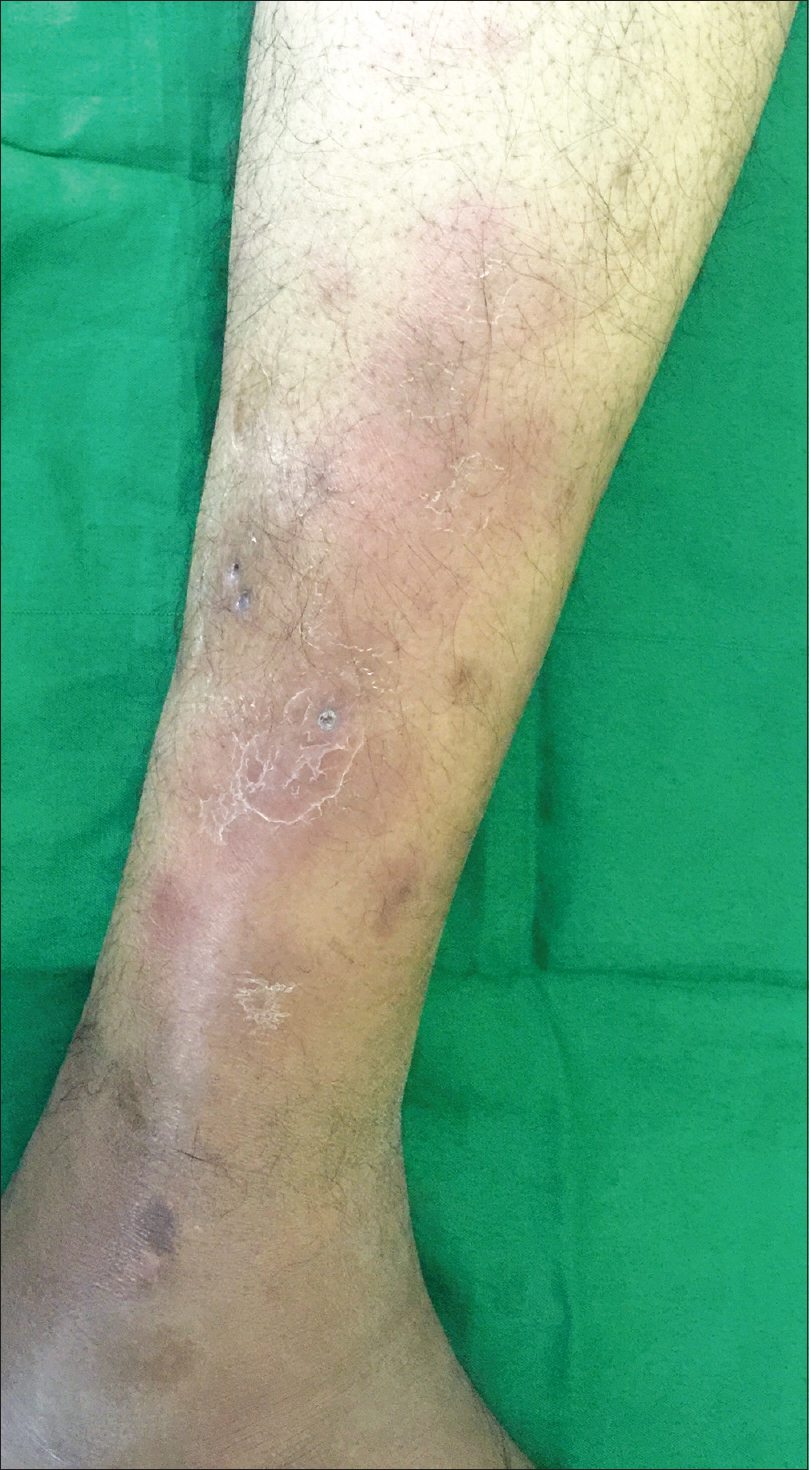 |
| Figure 1: Multiple erythematous nodules coalescing to form plaque over the leg |
An incisional skin biopsy obtained from one of the lesion showed infiltration of subcutaneous fat by atypical myeloid cells present predominantly in the septa [Figure 2a] and [Figure 2b]. The atypical cells were of myeloid lineage in different stages of maturation, including myeloblasts, myelocytes, metamyelocytes, and mature neutrophils with an admixture of variable number of lymphocytes and histiocytes. There was perivascular cuffing of atypical cells mostly around veins [Figure 2c]. There was evidence of infiltration by atypical cells in the venous wall along with endothelial swelling. The cells showed positivity for myeloperoxidase, confirming their myeloid lineage [Figure 2d] and were negative for CD3, CD20, and CD79a. Overall, features were of leukemic infiltration by chronic myeloid leukemia with septal panniculitis.
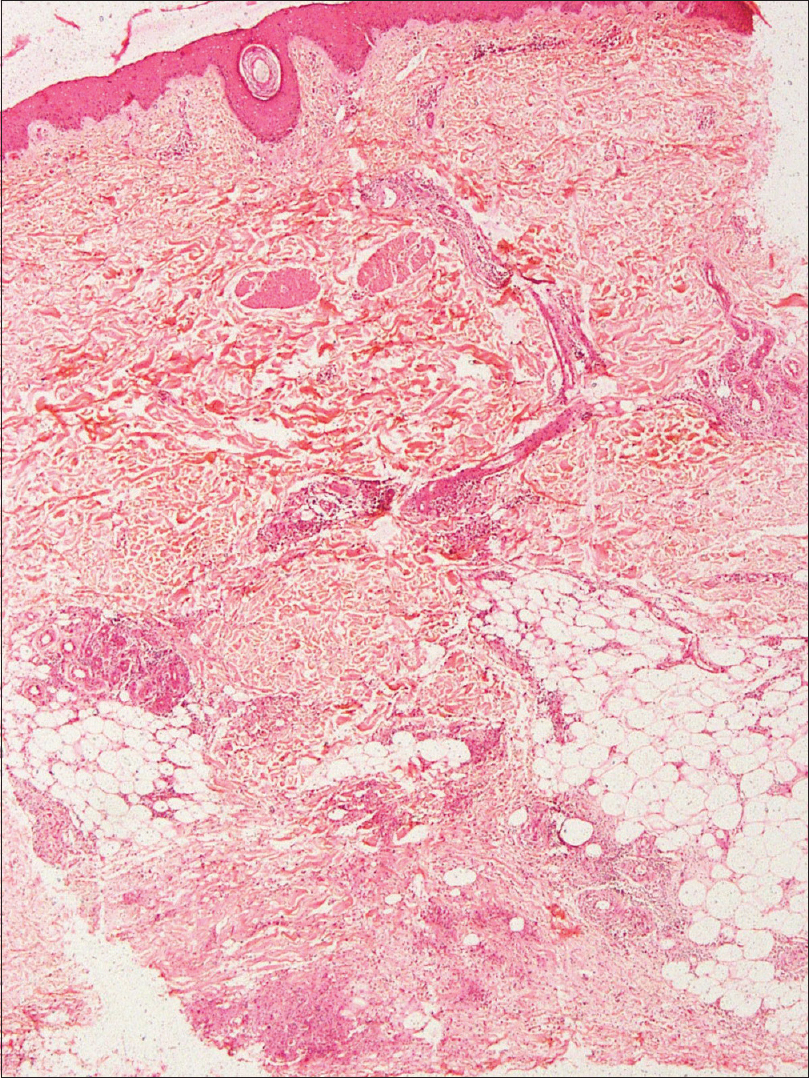 |
| Figure 2a: Moderately dense cellular infiltrate in the lower dermis and subcutaneous fat (H and E, ×400) |
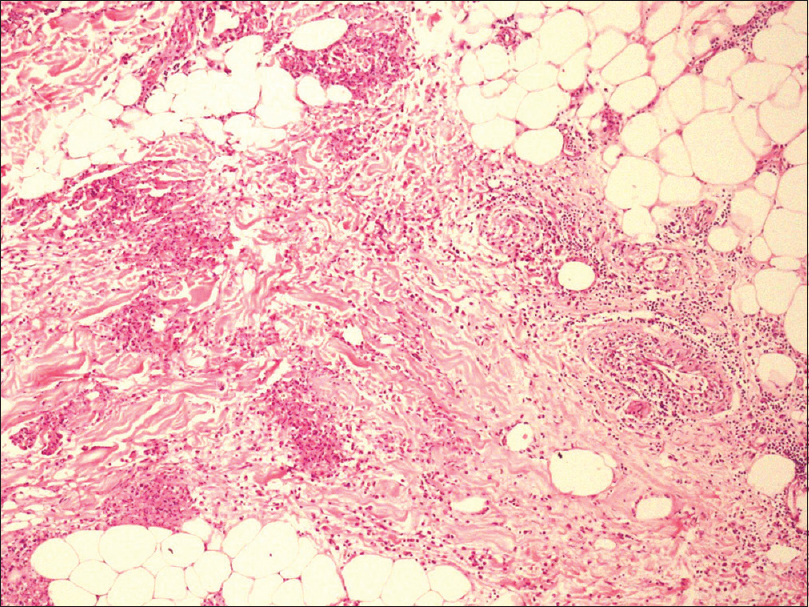 |
| Figure 2b: The infiltrate shows perivascular distribution with extension into the subcutaneous fat producing septal panniculitis (H and E, ×100) |
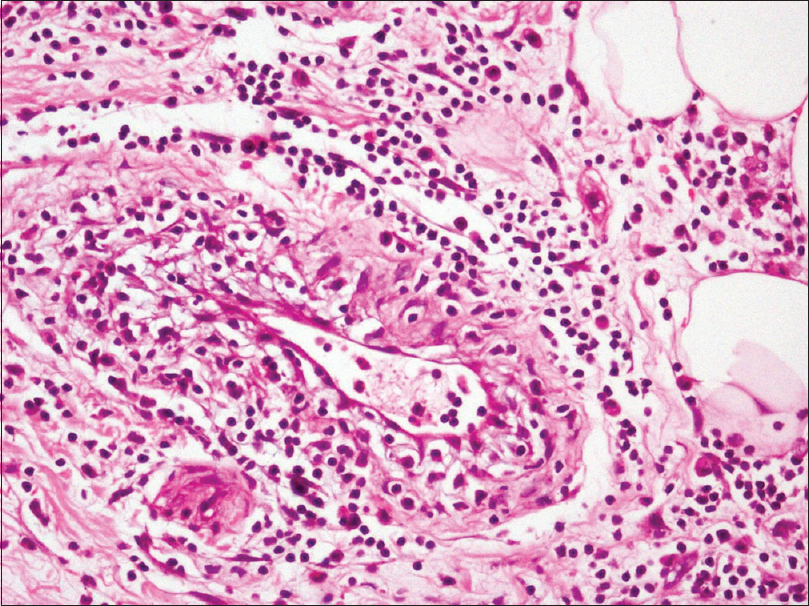 |
| Figure 2c: These cells were composed of myeloid precursors admixed with lymphocytes and histiocytes (H and E, ×400) |
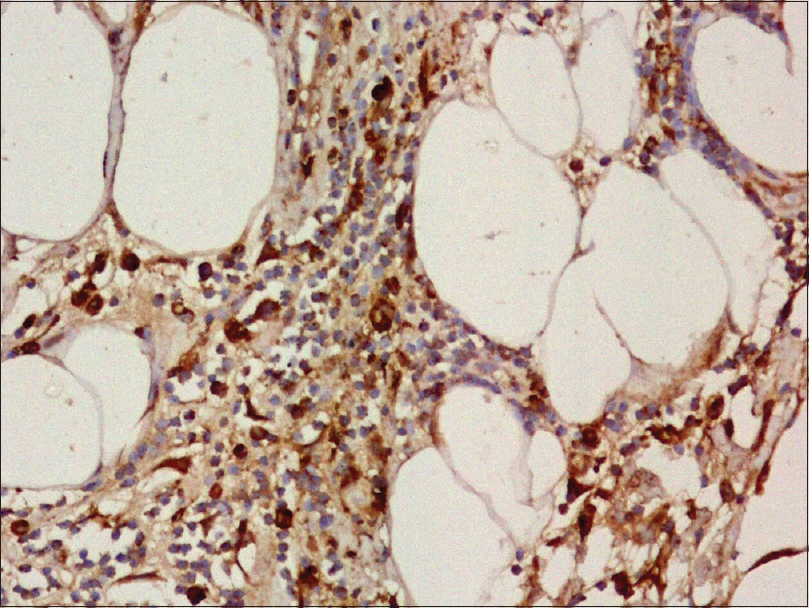 |
| Figure 2d: The immature myeloid cells show strong positivity for myeloperoxidase (×400) |
The patient's hemogram revealed hemoglobin of 10.4 g/dL, leucocyte count of 4.9 × 109/L with a normal differential, platelet count of 3.8 × 109/L, and no immature cells on the periphery. Bone marrow was not in hematological remission with 5% blasts, and reverse transcriptase-polymerase chain reaction test showed presence of the BCR-ABL fusion gene. He could not afford kinase domain mutation analysis or second-line tyrosine kinase inhibitors. As a palliative measure, he was treated with oral prednisolone in tapering doses with partial clinical improvement. He developed a rapidly rising leukocyte count (164.6 × 109/L) and myeloid blast crisis within 3 weeks of these skin lesions, suggesting that the skin lesions were the harbinger of the fatal chronic myeloid leukemia-blast crisis. The patient succumbed to his illness 3 months after his initial presentation.
Leukemia cutis has diverse clinical presentation, depending on whether leukemic cells infiltrate the epidermis, dermis, or subcutaneous fat. In general, the most common lesions in leukemia cutis tend to be multiple papules and nodules (60%) and infiltrated plaques (26%).[1] Erythema nodosum, a reactive panniculitis, is commonly caused by infections, drugs, systemic illnesses such as sarcoidosis and inflammatory bowel disease, pregnancy, and malignancy.[3] Erythema nodosum as a presenting manifestation of leukemia cutis is rarely reported in the literature.[4],[5],[6]
The leukemic infiltrates seen in our case represents a histological conundrum. In our patient, clinical presentation and certain histopathological features (septal panniculitis) suggested a diagnosis of erythema nodosum. In addition, preceding respiratory tract infection, concurrent malignancy, and imatinib intake are all known triggers of erythema nodosum.[3] Leukemic infiltration tends to preferentially occur at sites of previous or concomitant inflammation.[1],[2] Walther et al.[2] opined that tightly-cuffed perivascular, periadnexal, perineural, and nodular infiltrate of densely packed leukemic cells represent a reactive infiltrative process and differ from the neoplastic infiltrates of leukemia cutis which predominantly show interstitial infiltrate in the Indian filing pattern. Patel et al. voiced a similar opinion.[6] Although our patient had no circulating immature cells at presentation, in view of predominant perivascular and periadnexal infiltrate of leukemic cells, our case probably reflects a reactive process and not true leukemia cutis.
Histiocytoid Sweet's syndrome, which is characterized by the dermal infiltration of immature neutrophilic granulocytes, was considered in the histopathological differential of our patient. Subcutaneous variant of histiocytoid Sweet's syndrome can clinically simulate erythema nodosum.[7] The immature neutrophilic granulocytes in histiocytoid Sweet's syndrome are also myeloperoxidase positive, but differ from leukemia cutis by absence of tissue or circulating leukemic cells and lack of BCR/ABL fusion gene in the cells of the dermal infiltrate. Presence of frankly malignant cells in the tissue specimen excluded this possibility.
In conclusion, perivascular, periadnexal, perineural, and nodular infiltrate of densely packed leukemic cells represent a reactive infiltrative process and differs from leukemia cutis per se which shows interstitial infiltrate in the Indian filing pattern. Leukemic skin infiltrates in chronic myeloid leukemia patients portend a poor prognosis with aggressive course and reduced survival. It can resemble common dermatoses such as erythema nodosum and Sweet's syndrome, and histopathological evaluation is necessary to establish the true diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008;129:130-42.
[Google Scholar]
|
| 2. |
Walther BS, Gibbons G, Chan EF, Ziselman E, Rothfleisch JE, Willard RJ, et al. Leukemia cutis (involving chronic lymphocytic leukemia) within excisional specimens: A series of 6 cases. Am J Dermatopathol 2009;31:162-5.
[Google Scholar]
|
| 3. |
Blake T, Manahan M, Rodins K. Erythema nodosum – A review of an uncommon panniculitis. Dermatol Online J 2014;20:22376.
[Google Scholar]
|
| 4. |
Sumaya CV, Babu S, Reed RJ. Erythema nodosum-like lesions of leukemia. Arch Dermatol 1974;110:415-8.
[Google Scholar]
|
| 5. |
Matsuoka LY. Neoplastic erythema nodosum. J Am Acad Dermatol 1995;32(2 Pt 2):361-3.
[Google Scholar]
|
| 6. |
Patel RR, Kirkland EB, Nguyen DH, Cooper BW, Baron ED, Gilliam AC. Erythema nodosum in association with newly diagnosed hairy cell leukemia and group C streptococcus infection. Am J Dermatopathol 2008;30:160-2.
[Google Scholar]
|
| 7. |
Chow S, Pasternak S, Green P, Tremaine R, Reardon M, Murray S, et al. Histiocytoid neutrophilic dermatoses and panniculitides: Variations on a theme. Am J Dermatopathol 2007;29:334-41.
[Google Scholar]
|
Fulltext Views
4,457
PDF downloads
2,730





