Translate this page into:
Elimination of leprosy in India: An analysis
Correspondence Address:
Utpal Sengupta
Stanley Browne Laboratory, The Leprosy Mission Trust India, TLM Community Hospital, Nand Nagri, Shahdara, New Delhi - 110 093
India
| How to cite this article: Sengupta U. Elimination of leprosy in India: An analysis. Indian J Dermatol Venereol Leprol 2018;84:131-136 |
Abstract
India attained the elimination figure of less than 1 case of leprosy per 10,000 people during December 2005. Despite this, India still accounts for the largest number of new leprosy cases in the world, maintaining more than 50 per cent of the leprosy burden of the world, notwithstanding over three decades of use of multidrug therapy. The present review analyzes the process of execution of the elimination program, identifies any lacunae therein and presents corrective measures that could be taken up for elimination of the disease from the country.Introduction
Mycobacterium species are known to be commonly present in the environment, with Mycobacterium leprae as the first such organism to be to be associated with leprosy in humans, by G.A. Hansen in 1873.[1] However, in 2009, skeletal evidence for existence of leprosy in human civilization since 2000 BC was documented.[2] Molecular evidence for the presence of M. leprae in 10th century human skeletal remains has also been presented.[3] More than three decades ago when leprosy was endemic in many countries, India had more than 50% share of leprosy patients of the world. At present, in spite of availability and implementation of an effective multidrug therapy (MDT) for more than 30 years and attainment of elimination (<1 case/10,000 population size as defined by World Health Organization [WHO]) in 2002),[4] India continues to have a high share of 58.8% of the world leprosy population.[5] This might be due to the addition of new cases (who were incubating the disease) in the existing leprosy population. After the declaration of elimination, in 2005, of this chronic and long incubating disease, the vertical leprosy control program was quickly integrated with the general health services.[6] It can be seen from the trends in prevalence rate in India, that there was initially a steep fall in prevalence from 25.9/10,000 of 1991 to 5.9/10,000 in 1996, but thereafter, it gradually declined and showed a plateau from 2007 till 2016, even after attaining elimination.[7],[8] On the contrary, annual new case detection rate showed a gradual fall from 5.9/10,000 of 1991 to 2.3/10,000 in 2005, with rise to peaks in 1999 and 2000. However, between the years 2005 to 2015, both prevalence rate and annual new case detection rate remained in a plateau phase and the latter always exceeded the values of the former [Figure - 1]. This clearly indicates that although the number of cases as determined by prevalence rate has drastically gone down, the active transmission of infection has remained unchanged, as revealed by a steady level of annual new case detection rate [Figure - 1]. Thus, continuous occurrence of new cases in the population has greatly dampened the progress of National Leprosy Eradication Programme (NLEP). The remarkably steep decline in prevalence from 1991 to 2005 was due to wide coverage of the MDT program throughout the country, shortening of the drug regimen from 2 years to 1 year and designation of leprosy cases as cured cases when released from treatment.[9]
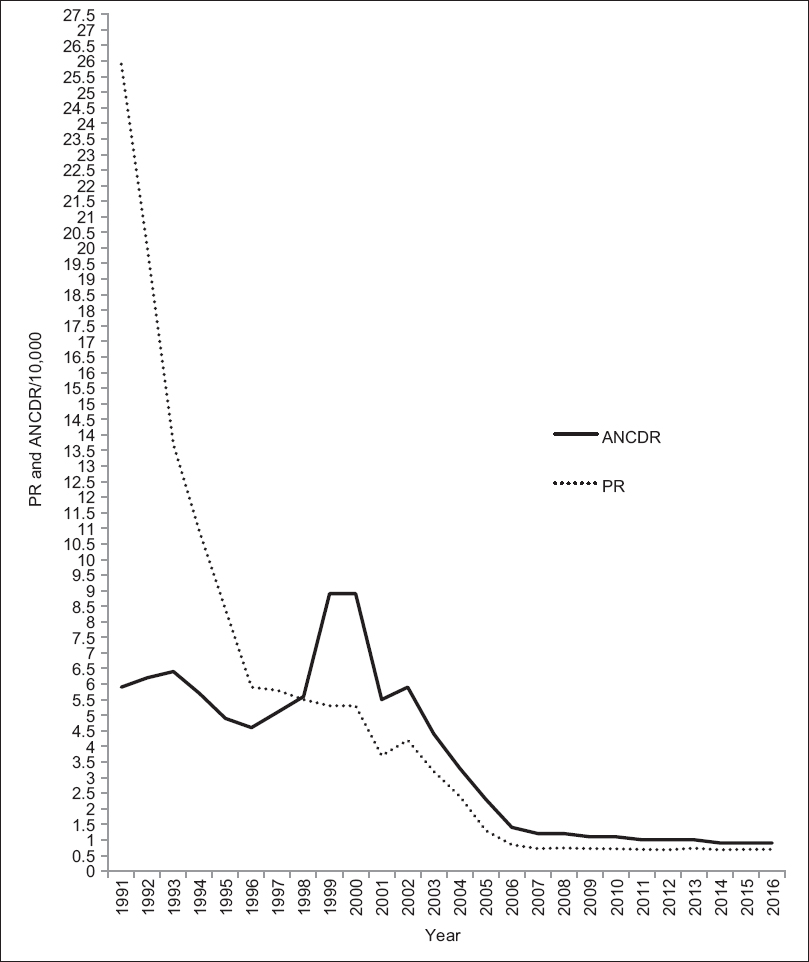 |
| Figure 1: Graph showing the trend of leprosy prevalence rate and annual new case detection rate from 1991 to 2016. ANCDR: Annual new case detection rate, PR: Prevalence rate |
To reduce annual new case detection rate, recently Government of India launched an active house-to-house survey in the form of Leprosy Case Detection Campaign and identified 31,666 active leprosy cases in the community, of which 3,755 cases were in the pediatric age group.[10]
The present analysis attempts to deal with the reasons for emergence of such a state during elimination era, wherein in spite of lowering of prevalence rate, new cases are appearing almost in the same numbers in the community. Further, the present review attempts to identify the reasons for transmission of leprosy in India during elimination era and suggests ways to adopt measures which will bring leprosy under control.
Reasons for active transmission
Long incubation period of the disease
It is known that the disease has a long incubation period, which may range from few weeks to 30 years.[11],[12],[13] Despite knowledge of this fact, the time taken for declaration of elimination, after attainment of prevalence rate < 1/10,000, was too short, which is revealed by the continuous emergence of new cases along with a rise in cases amongst children in India from 2006 till date,[14],[15] and consequently, accumulation of undetected new cases in the population.[16] Further, with the assistance from World Bank, the first phase of integration exercises of leprosy program with the general health services was immediately initiated in low endemic regions in 2001, which was continued later all over the country in a phased manner till 2004.[17],[18],[19] Before the integration of leprosy control program with the general health services, there should have been a stage of rigorous disease surveillance mechanism for at least 5–10 years after the declaration of elimination for recording and treatment of the new leprosy cases that would have emerged from the infected incubating population. It is known that in a chronic disease such as leprosy, many infected contacts in the population who are incubating the disease will emerge later as leprosy cases,[20] and hence during the post-elimination period, appearance of new cases will delay the progress towards eradication. As there was no surveillance mechanism in place after elimination to screen for new cases, therefore, more than 50% of the world leprosy population is still residing in India.[4]
Follow-up of drug trials for short duration
Most of the follow-up of the drug trials for determination of duration of MDT under the program were for short duration [21] and relapses were noted to be low.[22] However, when an MDT-administered cohort was followed up for 16 years in Cebu, the relapse rate increased to 4.6 percent.[23] Furthermore, anticipating a low relapse rate, MDT was recommended initially for 2 years' duration,[24] which was further reduced to 1 year MDT for multibacillary (MB) cases of leprosy.[25] Under the program, patients who relapsed with a positive bacteriological index (BI) in skin smears after stopping MDT were not immediately re-enrolled for treatment, leading to a delay in commencement of chemotherapy. This delay in re-enrollment of relapsed patients for re-treatment might have spread the infection in the community. Later, reports on relapse rates after long-term follow-up of MB cases were documented from many countries of the world.[26],[27],[28],[29] Well-controlled institutional studies from West Africa [30],[31] and India,[32],[33] including large number of cohorts, have reported significant number of relapses after 2 years of WHO regimen. However, reports were also available regarding the suitability of 2 years [34],[35] and 1 year [36] regimen from WHO MDT trials from India, Ethiopia and Cebu, wherein relapse rates were found to be very minimal.
The method of determination of bacterial killing by antileprosy drugs
As the morphological index did not correlate well with viability of M. leprae after administration of different dosages of antileprosy drugs,[37] efficacy of bacterial killing of M. leprae in patients under drug trials was mostly assessed by mouse foot pad growth of M. leprae inoculations isolated from biopsies of patients.[38] With this robust mouse foot pad assay for determination of growth, researchers have reported the presence of viable M. leprae after fixed dose therapy.[39],[40] Using other in vitro methods for assessment of viability, researchers could also show the presence of viable M. leprae after 2 years of MDT.[41]
Fixed dose therapy under NLEP for MB patients
It has been often noted that relapses are occurring more in MB patients after fixed dose therapy.[32],[33] Relapses have recently been observed more in highly bacillated (BI 3+ to 5+) MB patients.[42] It is of utmost importance to find out whether high BI patients at the time of stopping MDT harbor viable bacilli, which might lead to relapses. Recently, a molecular assay using real-time reverse transcription polymerase chain reaction (RT-PCR) has been developed for determining M. leprae viability in environmental or clinical samples based on 16S ribosomal RNA.[43] It has been noted using RT-PCR, that after fixed dose therapy, several copies of 16S ribosomal RNA remain in patients' tissues, indicating the presence of viable bacilli [Figure - 2] (under publication). As lepromatous cases specifically lack cell-mediated immunity to kill M. leprae, the left out viable bacilli which might remain silent will grow later either into drug-sensitive or drug-resistant M. leprae. A recent study has shown that M. leprae obtained from relapsed cases after 24 or 12 months fixed dose therapy are able to grow in mouse foot pad .[44] Therefore, by the time these relapsed cases are recognized, either in the field or in hospitals, they could have transmitted the infection to their household and neighboring contacts. Further, if the relapse occurs due to secondary resistance, then the relapsed patient will infect a naïve susceptible contact who might develop leprosy with a primary resistant strain of M. leprae in future.[45]
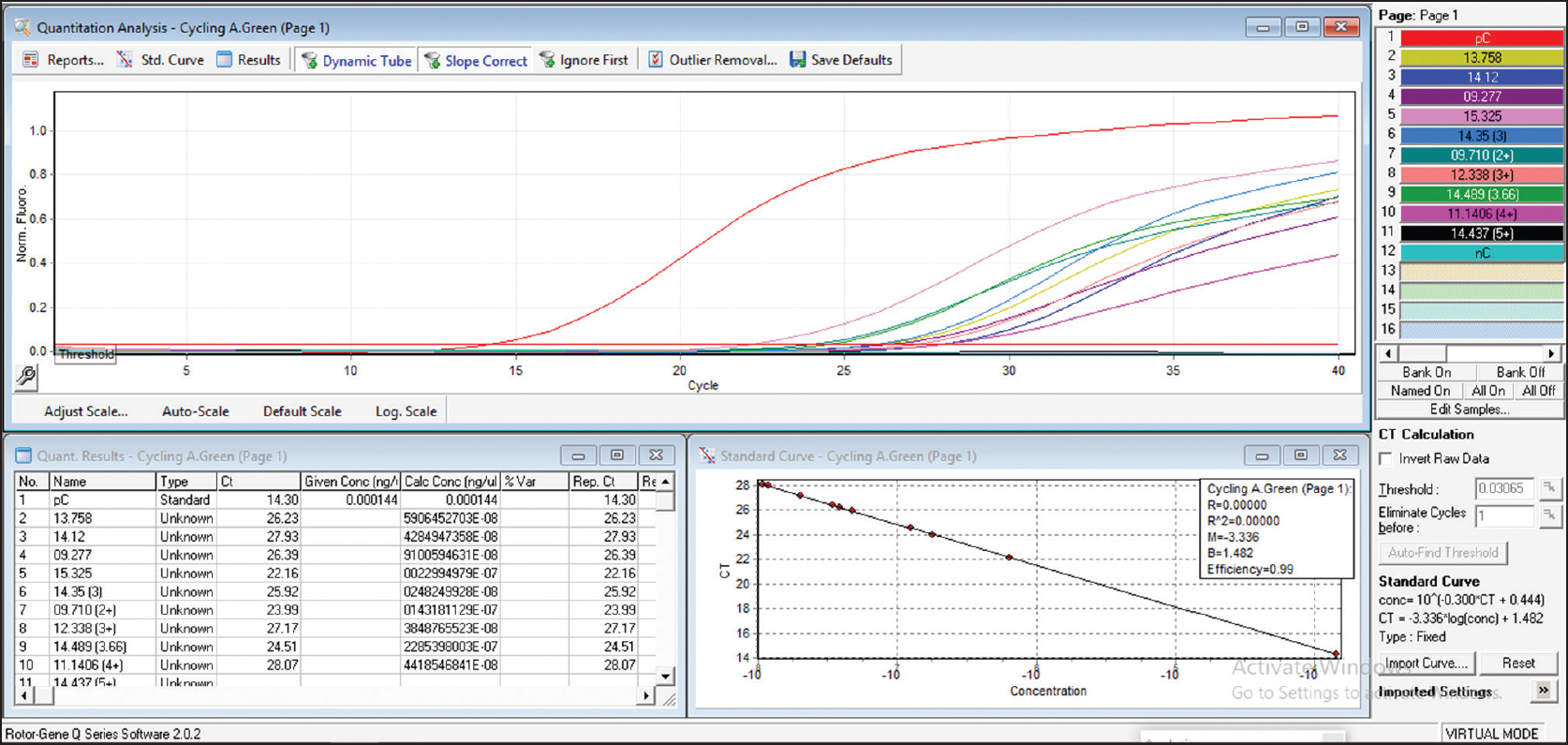 |
| Figure 2: Absolute quantification of copy number of 16S rRNA gene from skin smear scrapings of patients after completion of multidrug therapy regimen |
Quick integration of the NLEP with the general health services
As already stated above, after attainment of elimination figure of <1/10,000 in December 2005, there was a quick integration of the vertical NLEP program with the general health services.[5] A chronic disease, such as leprosy, which has a long incubation period is expected to have many individuals in the population who will be incubating the disease for many years before they become clinical cases. Therefore, a surveillance system should have been in place under the program for quick detection and treatment of new cases to halt transmission of infection in the community. As this surveillance mechanism was not in place, especially in endemic pockets, transmission of infection continued in the community.
Noncompliance to MDT
It has been revealed from several studies that a significant number of cases on treatment drop out from multidrug regimen due to several reasons.[46],[47],[48],[49],[50] These patients either do not take treatment until they clinically worsen, or go to some other private doctors who may not prescribe a proper antileprosy drug regimen. Hence, the patients lost to follow-up would have transmitted the disease in the community till they are again enrolled for administration of MDT under NLEP.
Health and hygiene
Most of the endemic pockets are now restricted to the states like Orissa, Bihar, Uttar Pradesh, Madhya Pradesh, West Bengal, Chhattisgarh, and Dadra Nagar Haveli where leprosy was endemic from the very beginning.[8] In these endemic areas, basic hygiene and sanitation, essential for a good healthcare system, is not available, especially in villages. In most of these rural areas, the source of water is from ponds which are used for daily washing and bathing purposes by all inhabitants along with the patients. In such a situation where there is clustering of active cases, the contacts are getting constantly exposed directly to both patients and an environment (soil and water) wherein M. leprae has been shown to remain alive in all seasons for long duration.[51],[52] Therefore, if the environment for health and hygiene is not improved, then shedding of bacilli from infected cases will prove to be a continuous source of exposure to infection.
Action plan to halt transmission
The diagnosis and treatment of active cases in the community
There is an immediate need for implementation of house-to-house surveys on a war footing in diagnosing the active cases and enrolling them immediately under MDT program. It is to be mentioned that India has already initiated the same by implementation of leprosy case detection campaign program in endemic districts throughout the country. Under this program, house-to-house survey in endemic villages of most of the states has been initiated.[53] It is anticipated that this approach when implemented throughout the country will bring almost all the active cases under treatment, and will bring down the number of these cases to a great extent. However, a surveillance mechanism has to be sustained, to serve as “watch dog” for regular screening of contacts in the population for new cases after the completion of leprosy case detection campaign.
The protection of contacts of index cases from getting M. leprae infection
Immediate steps have to be undertaken to reduce the exposure time of contacts with the index cases. To achieve this, one dose of rifampicin administration to all contacts of index cases has been initiated by NLEP while implementing leprosy case detection campaign in most of the endemic districts of the country. This strategy has been adopted because chemoprophylaxis of contacts with one dose of rifampicin has been found to be very effective in reducing the transmission of infection in COLEP trial in Bangladesh. This double-blind trial has shown a significant protection from clinical leprosy in the contact population at Bangladesh up to 2 years.[54] Earlier, during the dapsone monotherapy regimen between 1960 and 1970, chemoprophylaxis of contacts with twice weekly doses of dapsone administration for many years was adopted by several countries which showed significant protection against leprosy. It was also noted that the efficacy of protection was higher at the community level compared to that of the household contacts, although the number of non-household contacts to be treated was much higher.[55] Almost at the same time dapsone resistance emerged in the population.[56],[57] However, an immediate worldwide implementation of administration of MDT for treatment of leprosy recommended by WHO controlled the situation of dapsone resistance.[58] It may be pointed out that dapsone-resistant M. leprae emerged almost 30 years after dapsone monotherapy. Now that more than another three decades have passed with administration of rifampicin in MDT drug regimen, it is expected that M. leprae, although a slow growing bacteria, will start showing up changes in its genes with mutation leading to evolution of rifampicin-resistant strains. Rifampicin resistance has already been reported from different endemic regions of India.[45] Considering the above, especially when secondary rifampicin resistance is being reported from various endemic regions of leprosy whether single-dose rifampicin for chemoprophylaxis will be a wise proposition for reduction in transmission of infection will be revealed only from the future survey of contacts of index cases who will be subjected to chemoprophylaxis under the control program.
Reduction in default rate
Default in treatment has been one of the major issues for failure of the program. The health workers need to be trained to find out about the patients' regularity of intake of medicine, ensuring that they do not default from the MDT regimen. This would require continuous monitoring of patients by healthcare workers to remind them to take medicines as per instructions. The mechanism for the same may be evolved using mobile networking system.
Contact Tracing
It has been shown in India that contact tracing- examination of persons having contact with a leprosy patient- is a very important component to identify populations at high risk for developing leprosy.[59],[60],[61] Contact tracing along with chemoprophylaxis with rifampicin was applied by WHO under COLEP trial in Bangladesh to reduce transmission of leprosy.[54] Therefore, contact tracing and their examination for early disease manifestations could be introduced immediately under NLEP. Health workers could be trained so that the patients get committed to bring their contacts to the primary health center for clinical examination.
Reduction in stigma
Stigma in leprosy is known to cause social discrimination. Due to this fear of discrimination, patients frequently hide early symptoms and signs of leprosy [62] and therefore, are not treated at a proper time. This delay might be responsible for spreading the infection to their healthy contacts. After initiation of treatment, often patients do not report to the general health services or the tertiary care hospital because of fear of rejection by society and their community, and therefore, in certain countries non-compliance to treatment may go up to 40 percent.[63]
Lack of knowledge regarding signs and symptoms of leprosy in the community
To achieve a success in any control program of a disease, it is of prime importance that people in the community should be aware about the early signs and symptoms of the disease. Several studies carried out in endemic countries indicate that knowledge in the community about signs and symptoms of leprosy is inadequate.[64],[65] Therefore, there is an immediate need for education of the community regarding early signs and symptoms of leprosy so that there is a rise in perception and awareness of the disease.
Immunomodulation
An immunomodulator that will be able to upgrade the required cell-mediated immunity against M. leprae in a susceptible host will definitely be useful in reducing the transmission of the disease. It has been well established that a vaccine that would protect 60–90% of the population from getting infected is very useful to reduce transmission for infectious diseases.[66],[67] BCG, has already been found to provide increased protection to the contact population from 50% in Malawi [68],[69] to 90% in Sao Paulo, Brazil.[70] Another vaccine earlier named Mw and now categorized as M. indicus pranii, a saprophyte, sharing genes with both M. leprae and M. tuberculosis,[71] was also tried for determining its efficacy in protecting against leprosy infection. It was observed that M. indicus pranii-vaccinatedpopulation was protected up to 68%, 60%, and 28% at the end of the first, second, and third year post-vaccination, respectively. As this is a heat-killed bacteria, it required a booster dose after 2 years to restore the immunity.[72] Hence, such a vaccine having immunomodulating properties may be useful in curtailing transmission of leprosy.
Conclusion
This article has briefly described the present scenario of leprosy burden, action executed by Government of India, the reasons for continuous transmission of leprosy and the measures to circumvent active transmission of leprosy in the country. It has pointed out the preventive approach to be considered under the elimination program and also has described the measures to control the disease both at the community and at the national level.
Acknowledgements
The author is grateful to the Director, The Leprosy Mission Trust India for his help. Dr. Ravindra P. Turankar and Dr. Itu Singh, Research Scientists have helped in discussion and data compilation of the manuscript. The author also acknowledges Dr. Mallika Lavania for providing the preliminary data on real-time RT-PCR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Irgens LM. The discovery of the leprosy bacillus. Tidsskr Nor Laegeforen 2002;122:708-9.
[Google Scholar]
|
| 2. |
Robbins G, Tripathy VM, Misra VN, Mohanty RK, Shinde VS, Gray KM, et al. Ancient skeletal evidence for leprosy in India (2000 B.C.). PLoS One 2009;4:e5669.
[Google Scholar]
|
| 3. |
Haas CJ, Zink A, Pálfi G, Szeimies U, Nerlich AG. Detection of leprosy in ancient human skeletal remains by molecular identification of Mycobacterium leprae. Am J Clin Pathol 2000;114:428-36.
[Google Scholar]
|
| 4. |
World Health Organization. Global Target Attained. Remaining Endemic Countries Pose Greatest Challenge. Press Release, WHA/2. 16th May, 2002. World Health Organization; 2002.
[Google Scholar]
|
| 5. |
Global leprosy update, 2014: Need for early case detection. Wkly Epidemiol Rec 2015;90:461-74.
[Google Scholar]
|
| 6. |
National Leprosy Eradication Programme (NLEP). India Achieved Elimination of Leprosy; December, 2005. Available from: http://www.mohfw.nic./in/data2html. [Last accessed on 2017 Jan 24].
[Google Scholar]
|
| 7. |
Central Leprosy Division-NLEP. Available from: http://www.nlep.nic.in/pdf/ProgressReport2011-11. [Last accessed on 2017 Jan 13].
[Google Scholar]
|
| 8. |
Central Leprosy Division-NLEP. Available from: http://www.nlep.nic.in/pdf/ProgressReport2014-15. [Last accessed on 2017 Jan 13].
[Google Scholar]
|
| 9. |
Saunderson PR. Leprosy elimination: Not as straightforward as it seemed. Public Health Rep 2008;123:213-6.
[Google Scholar]
|
| 10. |
Kumar A. 31,666 'hidden' leprosy cases found in door to door survey. Indian Express Newspaper. Mumbai Edition. 21st November, 2016.
[Google Scholar]
|
| 11. |
Joshi PL. Epidemiology of leprosy. In: Kar HK, Kumar B, editors. IAL Textbook of Leprosy. 2nd ed. New Delhi: Jaypee Brothers; 2016. p. 33-44.
[Google Scholar]
|
| 12. |
Girdhar A, Mishra B, Lavania RK, Bagga AK, Malaviya GN, Girdhar BK, et al. Leprosy in infants – Report of two cases. Int J Lepr Other Mycobact Dis 1989;57:472-5.
[Google Scholar]
|
| 13. |
Pinheiro RO, de Souza Salles J, Sarno EN, Sampaio EP. Mycobacterium leprae-host-cell interactions and genetic determinants in leprosy: An overview. Future Microbiol 2011;6:217-30.
[Google Scholar]
|
| 14. |
Leprosy. Global target attained. Wkly Epidemiol Rec 2001;76:155-6.
[Google Scholar]
|
| 15. |
Singal A, Sonthalia S. Leprosy in post-elimination era in India: Difficult journey ahead. Indian J Dermatol 2013;58:443-6.
[Google Scholar]
|
| 16. |
Kumar A, Girdhar A, Chakma JK, Girdhar BK. Detection of previously undetected leprosy cases in Firozabad district (U.P.), India during 2006-2009: A short communication. Lepr Rev 2013;84:124-7.
[Google Scholar]
|
| 17. |
Pandey A, Patel R, Uddin MJ. Leprosy control activities in India: Integration into general health system. Lepr Rev 2006;77:210-8.
[Google Scholar]
|
| 18. |
Pandey A, Rathod H. Integration of leprosy into GHS in India: A follow up study (2006-2007). Lepr Rev 2010;81:306-17.
[Google Scholar]
|
| 19. |
Byamungu DC, Ogbeiwi OI. Integrating leprosy control into general health service in a war situation: The level after 5 years in Eastern Congo. Lepr Rev 2003;74:68-78.
[Google Scholar]
|
| 20. |
Fine PE, Sterne JA, Pönnighaus JM, Bliss L, Saui J, Chihana A, et al. Household and dwelling contact as risk factors for leprosy in Northern Malawi. Am J Epidemiol 1997;146:91-102.
[Google Scholar]
|
| 21. |
Fajardo TT, Villahermosa L, Pardillo FE, Abalos RM, Burgos J, Dela Cruz E, et al. A comparative clinical trial in multibacillary leprosy with long-term relapse rates of four different multidrug regimens. Am J Trop Med Hyg 2009;81:330-4.
[Google Scholar]
|
| 22. |
Risk of relapse in leprosy. The Leprosy Unit, WHO. Indian J Lepr 1995;67:13-26.
[Google Scholar]
|
| 23. |
Balagon MF, Cellona RV, Cruz Ed, Burgos JA, Abalos RM, Walsh GP, et al. Long-term relapse risk of multibacillary leprosy after completion of 2 years of multiple drug therapy (WHO-MDT) in Cebu, Philippines. Am J Trop Med Hyg 2009;81:895-9.
[Google Scholar]
|
| 24. |
Chemotherapy of leprosy. Report of a WHO study group. World Health Organ Tech Rep Ser 1994;847:1-24.
[Google Scholar]
|
| 25. |
Ji B. Why multidrug therapy for multibacillary leprosy can be shortened to 12 months. Lepr Rev 1998;69:106-9.
[Google Scholar]
|
| 26. |
Balagon MF, Cellona, RV, Fajardo TT, Villahermosa LG, Dela Cruz EC, Tan EV et al. Relapses in multibacillary leprosy after 2 years treatment with WHO-MDT regimen. XV International Leprosy Conference, Beijing, China. Int J Lepr 1998;66:7A.
[Google Scholar]
|
| 27. |
Shetty VP, Dighe RR, Uplekar MW, Antia NH, Pai VV, Ganapati R. Relapse and recurrence of lesions after MDT in leprosy: Clinical, bacteriological and histopathological investigations of 56 cases. XV International Leprosy Conference, Beijing, China. Int J Lepr 1998;66:7A.
[Google Scholar]
|
| 28. |
Jian D, Hu L, Luo, J. A long term observation on the effect of multi drug therapy in leprosy in Liang Shan and Pangzhhua. XV International Leprosy Conference, Beijing, China. Int J Lepr 1998;66:10A.
[Google Scholar]
|
| 29. |
Ebenzer GJ, Barkataki A. Relapsed leprosy after multidrug therapy presenting as histoid form. XV International Leprosy Conference, Beijing, China. Int J Lepr 1998;66:7A.
[Google Scholar]
|
| 30. |
Jamet P, Ji B. Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Marchoux chemotherapy study group. Int J Lepr Other Mycobact Dis 1992;60:525-35.
[Google Scholar]
|
| 31. |
Jamet P, Ji B. Relapse after long-term follow up of multibacillary patients treated by WHO multidrug regimen. Marchoux chemotherapy study group. Int J Lepr Other Mycobact Dis 1995;63:195-201.
[Google Scholar]
|
| 32. |
Girdhar BK, Girdhar A, Kumar A. Relapses in multibacillary leprosy patients: Effect of length of therapy. Lepr Rev 2000;71:144-53.
[Google Scholar]
|
| 33. |
Shaw IN, Christian M, Jesudasan K, Kurian N, Rao GS. Effectiveness of multidrug therapy in multibacillary leprosy: A long-term follow-up of 34 multibacillary leprosy patients treated with multidrug regimens till skin smear negativity. Lepr Rev 2003;74:141-7.
[Google Scholar]
|
| 34. |
Vijayakumaran P, Jesudasan K, Manimozhi N. Fixed-duration therapy (FDT) in multibacillary leprosy; efficacy and complications. Int J Lepr Other Mycobact Dis 1996;64:123-7.
[Google Scholar]
|
| 35. |
Gebre S, Saunderson P, Byass P. Relapses after fixed duration multiple drug therapy: The AMFES cohort. Lepr Rev 2000;71:325-31.
[Google Scholar]
|
| 36. |
Maghanoy A, Mallari I, Balagon M, Saunderson P. Relapse study in smear positive multibacillary (MB) leprosy after 1 year WHO-multi-drug therapy (MDT) in Cebu, Philippines. Lepr Rev 2011;82:65-9.
[Google Scholar]
|
| 37. |
Waters MF, Rees RJ. Changes in the morphology of Mycobacterium leprae in patients under treatment. Int J Lepr 1962;30:266-77.
[Google Scholar]
|
| 38. |
Levy L. The THELEP Controlled Clinical Trials in Lepromatous Leprosy. TDR/IDE/THELEP/99.1. Geneva, Switzerland: World Health Organization; 1999. p. 93.
[Google Scholar]
|
| 39. |
Shetty VP, Wakade AV, Ghate S, Pai VV, Ganapati R, Antia NH, et al. Viability and drug susceptibility testing of M. leprae using mouse footpad in 37 relapse cases of leprosy. Int J Lepr Other Mycobact Dis 2003;71:210-7.
[Google Scholar]
|
| 40. |
Shetty VP, Suchitra K, Uplekar MW, Antia NH. Higher incidence of viable Mycobacterium leprae within the nerve as compared to skin among multibacillary leprosy patients released from multidrug therapy. Lepr Rev 1997;68:131-8.
[Google Scholar]
|
| 41. |
Katoch VM, Katoch K, Ramanathan U, Sharma VD, Shivannavar CT, Datta AK, et al. Effect of chemotherapy on viability of Mycobacterium leprae as determined by ATP content, morphological index and FDA-EB fluorescent staining. Int J Lepr Other Mycobact Dis 1989;57:615-21.
[Google Scholar]
|
| 42. |
Lavania M, Jadhav RS, Chaitanya VS, Turankar R, Selvasekhar A, Das L, et al. Drug resistance patterns in Mycobacterium leprae isolates from relapsed leprosy patients attending The Leprosy Mission (TLM) hospitals in India. Lepr Rev 2014;85:177-85.
[Google Scholar]
|
| 43. |
Martinez AN, Lahiri R, Pittman TL, Scollard D, Truman R, Moraes MO, et al. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. J Clin Microbiol 2009;47:2124-30.
[Google Scholar]
|
| 44. |
Shetty VP, Wakade AV, Ghate SD, Pai VV, Ganapati RR, Antia NH, et al. Clinical, histopathological and bacteriological study of 52 referral MB cases relapsing after MDT. Lepr Rev 2005;76:241-52.
[Google Scholar]
|
| 45. |
Lavania M, Nigam A, Turankar RP, Singh I, Gupta P, Kumar S, et al. Emergence of primary drug resistance to rifampicin in Mycobacterium leprae strains from leprosy patients in India. Clin Microbiol Infect 2015;21:e85-6.
[Google Scholar]
|
| 46. |
Rao PS. A study on non-adherence to MDT among leprosy patients. Indian J Lepr 2008;80:149-54.
[Google Scholar]
|
| 47. |
Honrado ER, Tallo V, Balis AC, Chan GP, Cho SN. Noncompliance with the World Health Organization-multidrug therapy among leprosy patients in Cebu, Philippines: Its causes and implications on the leprosy control program. Dermatol Clin 2008;26:221-9, vi.
[Google Scholar]
|
| 48. |
Heukelbach J, André Chichava O, de Oliveira AR, Häfner K, Walther F, de Alencar CH, et al. Interruption and defaulting of multidrug therapy against leprosy: Population-based study in Brazil's Savannah Region. PLoS Negl Trop Dis 2011;5:e1031.
[Google Scholar]
|
| 49. |
Kar S, Pal R, Bharati DR. Understanding non-compliance with WHO-multidrug therapy among leprosy patients in Assam, India. J Neurosci Rural Pract 2010;1:9-13.
[Google Scholar]
|
| 50. |
Girão RJ, Soares NL, Pinheiro JV, Oliveira Gda P, de Carvalho SM, de Abreu LC, et al. Leprosy treatment dropout: A systematic review. Int Arch Med 2013;6:34.
[Google Scholar]
|
| 51. |
Lavania M, Katoch K, Katoch VM, Gupta AK, Chauhan DS, Sharma R, et al. Detection of viable Mycobacterium leprae in soil samples: Insights into possible sources of transmission of leprosy. Infect Genet Evol 2008;8:627-31.
[Google Scholar]
|
| 52. |
Lavania M, Turankar RP, Karri S, Chaitanya VS, Sengupta U, Jadhav RS, et al. Cohort study of the seasonal effect on nasal carriage and the presence of Mycobacterium leprae in an endemic area in the general population. Clin Microbiol Infect 2013;19:970-4.
[Google Scholar]
|
| 53. |
Gitte SV. LCDC (Leprosy Cases Detection Campaign) – Aiming at cent percent detection of leprosy cases in the community based on a line of pulse polio campaign in leprosy endemic state. Int J Dermatol Clin Res 2016;2:12-3.
[Google Scholar]
|
| 54. |
Moet FJ, Pahan D, Oskam L, Richardus JH, COLEP Study Group. Effectiveness of single dose rifampicin in preventing leprosy in close contacts of patients with newly diagnosed leprosy: Cluster randomised controlled trial. BMJ 2008;336:761-4.
[Google Scholar]
|
| 55. |
Smith CM, Smith WC. Chemoprophylaxis is effective in the prevention of leprosy in endemic countries: A systematic review and meta-analysis. MILEP2 study group. Mucosal immunology of leprosy. J Infect 2000;41:137-42.
[Google Scholar]
|
| 56. |
Pettit JH, Rees RJ, Ridley DS. Studies on sulfone resistance in leprosy. I. Detection of cases. Int J Lepr Other Mycobact Dis 1966;34:375-90.
[Google Scholar]
|
| 57. |
Pearson JM, Rees RJ, Waters MF. Sulphone resistance in leprosy. A review of one hundred proven clinical cases. Lancet 1975;2:69-72.
[Google Scholar]
|
| 58. |
Chemotherapy of leprosy for control programmes. World Health Organ Tech Rep Ser 1982;675:1-33.
[Google Scholar]
|
| 59. |
Jesudasan K, Bradley D, Smith PG, Christian M. Incidence rates of leprosy among household contacts of “primary cases”. Indian J Lepr 1984;56:600-14.
[Google Scholar]
|
| 60. |
Sundar Rao PS, Jesudasan K, Mani K, Christian M. Impact of MDT on incidence rates of leprosy among household contacts. Part 1. Baseline data. Int J Lepr Other Mycobact Dis 1989;57:647-51.
[Google Scholar]
|
| 61. |
Vijayakumaran P, Jesudasan K, Mozhi NM, Samuel JD. Does MDT arrest transmission of leprosy to household contacts? Int J Lepr Other Mycobact Dis 1998;66:125-30.
[Google Scholar]
|
| 62. |
Bekri W, Gebre S, Mengiste A, Saunderson PR, Zewge S. Delay in presentation and start of treatment in leprosy patients: A case-control study of disabled and non-disabled patients in three different settings in Ethiopia. Int J Lepr Other Mycobact Dis 1998;66:1-9.
[Google Scholar]
|
| 63. |
de Stigter DH, de Geus L, Heynders ML. Leprosy: Between acceptance and segregation. Community behaviour towards persons affected by leprosy in Eastern Nepal. Lepr Rev 2000;71:492-8.
[Google Scholar]
|
| 64. |
Barkataki P, Kumar S, Rao PS. Knowledge of and attitudes to leprosy among patients and community members: A comparative study in Uttar Pradesh, India. Lepr Rev 2006;77:62-8.
[Google Scholar]
|
| 65. |
Tesema AA, Beriso M. Assessment of knowledge and attitude of community on leprosy patients in Kuyera Town, West Arsi Zone, Oromia Region Southeast Ethiopia. Hered Genet 2015;5:156.
[Google Scholar]
|
| 66. |
Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ 2008;86:140-6.
[Google Scholar]
|
| 67. |
Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis 2012;12:36-44.
[Google Scholar]
|
| 68. |
Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Karonga Prevention Trial Group. Lancet 1996;348:17-24.
[Google Scholar]
|
| 69. |
Pönnighaus JM, Fine PE, Sterne JA, Wilson RJ, Msosa E, Gruer PJ, et al. Efficacy of BCG vaccine against leprosy and tuberculosis in Northern Malawi. Lancet 1992;339:636-9.
[Google Scholar]
|
| 70. |
Lombardi C, Pedrazzani ES, Pedrazzani JC, Filho PF, Zicker F. Protective efficacy of BCG against leprosy in São Paulo. Bull Pan Am Health Organ 1996;30:24-30.
[Google Scholar]
|
| 71. |
Saini V, Raghuvanshi S, Talwar GP, Ahmed N, Khurana JP, Hasnain SE, et al. Polyphasic taxonomic analysis establishes Mycobacterium indicus pranii as a distinct species. PLoS One 2009;4:e6263.
[Google Scholar]
|
| 72. |
Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, Parida SK, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr Rev 2005;76:127-43.
[Google Scholar]
|
Fulltext Views
11,292
PDF downloads
4,193





