Translate this page into:
Calciphylaxis as cutaneous marker of hyperparathyroidism and successful outcome with parathyroidectomy followed by hyperbaric oxygen therapy
Correspondence Address:
Manas Chatterjee
Department of Dermatology, INHS Asvini, Colaba, Mumbai - 400 005, Maharashtra
India
| How to cite this article: Hemdani R, Rajput G R, Sridhar J, Chatterjee M, Rathod D. Calciphylaxis as cutaneous marker of hyperparathyroidism and successful outcome with parathyroidectomy followed by hyperbaric oxygen therapy. Indian J Dermatol Venereol Leprol 2018;84:209-211 |
Sir,
Calciphylaxis is a rare life-threatening syndrome characterized by progressive and painful skin ulcerations associated with calcification of medium-size and small cutaneous arteries. The term calciphylaxis was coined in 1962 by Selye.[1] It is also known as calcific uremic arteriopathy and “vascular calcification–cutaneous necrosis syndrome.”[2]
It mostly affects end-stage renal disease patients on dialysis. Incidence is in the range of 1:1000 to 1:1500 cases in patients on chronic renal replacement therapy.[3] Exceptions have been reported in patients with normal renal function and in association with chronic inflammatory disease, malignancy or primary hyperparathyroidism. Secondary infection of necrotic skin lesions and subsequent sepsis can cause high mortality of up to 80%.
A 24-year-old female with no known comorbidities presented with 10 days history of extreme pain on outer aspect of both thighs. Patient was unable to walk due to severe pain in the thighs. There was no history of loss of appetite, nausea, vomiting, constipation, abdominal pain, increased thirst, frequent urination, fatigue, weakness, muscle pain, confusion, disorientation, headache and depression. There was no history of drug intake, calcium or vitamin D supplementation in the past. The patient denied consuming alcohol or smoking and did not give any history suggestive of a connective tissue disease. On examination, our patient was thin, had low-grade fever, tachycardia and pallor. Dermatological examination revealed irregularly shaped, well-defined, indurated erythematous, and tender plaques measuring approximately 8 cm × 5 cm over lateral aspect of upper one-third of both thighs. These lesions increased in size and number over a span of 1 week [Figure - 1]a.
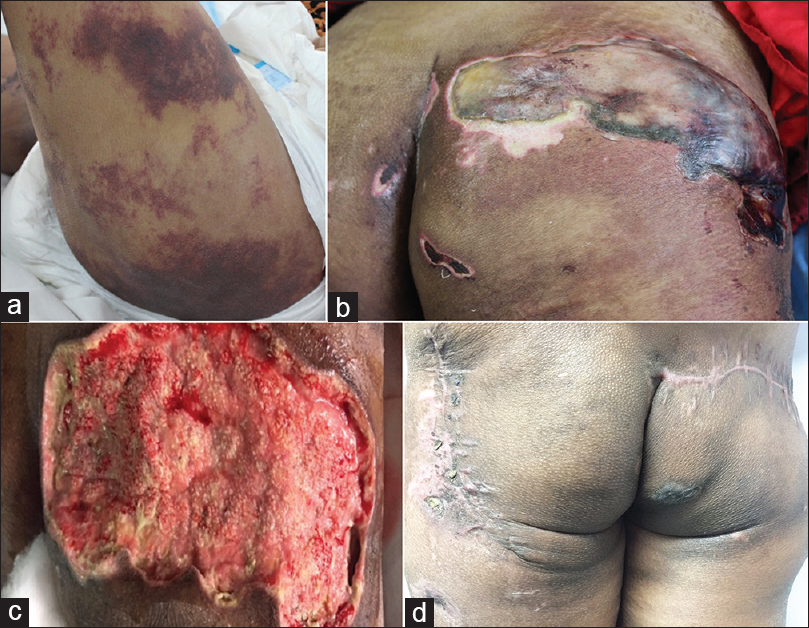 |
| Figure 1: (a) Erythematous tender plaques over the posterior-lateral aspect of left thigh (b) Hemorrhagic infarcts and necrotic eschar with involvement of right gluteal region (c) Healthy granulation tissue post hyperbaric oxygen therapy (d) Healthy scar at 16 weeks post primary closure of wound |
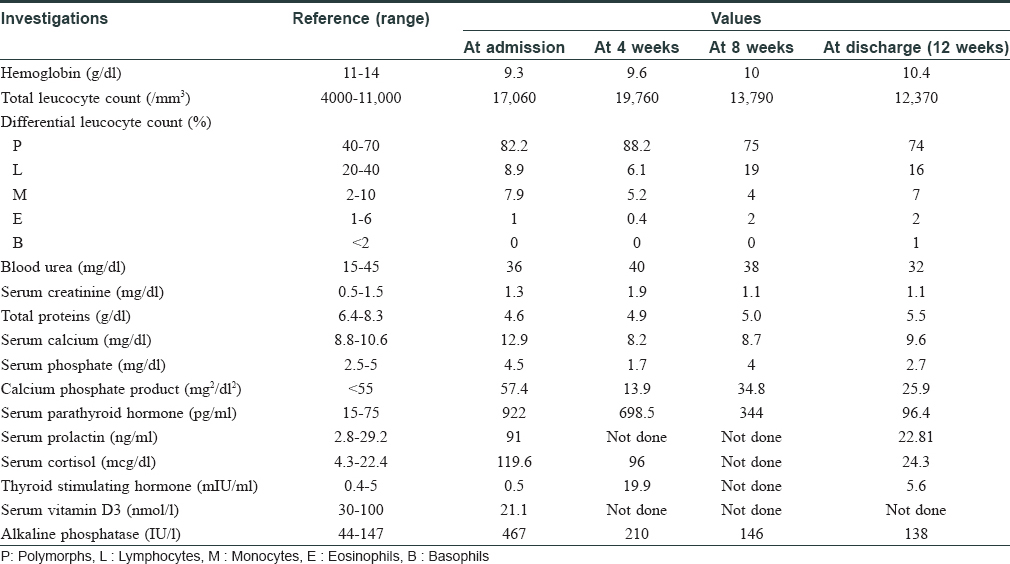
Investigations revealed anemia, leukocytosis, elevated serum calcium, alkaline phosphatase, parathyroid hormone, serum cortisol, serum prolactin and insufficient vitamin D3 as depicted serially in [Table - 1]. Technetium (99m Tc) SestaMIBI (sestamethoxyisobutylisonitrile) scan revealed left inferior parathyroid adenoma. A possibility of multiple endocrine neoplasia was kept but magnetic resonance imaging of brain was deferred due to nonavailability of this facility at our center at that time. The same was done later during follow-up and was found to be normal. Histopathology of lesion showed ulcerated epidermis with calcification and intimal hyperplasia in the small-sized cutaneous arterioles in hematoxylin and eosin stain. Calcium was demonstrated in cutaneous arteriole by Von Kossa stain [Figure - 2].
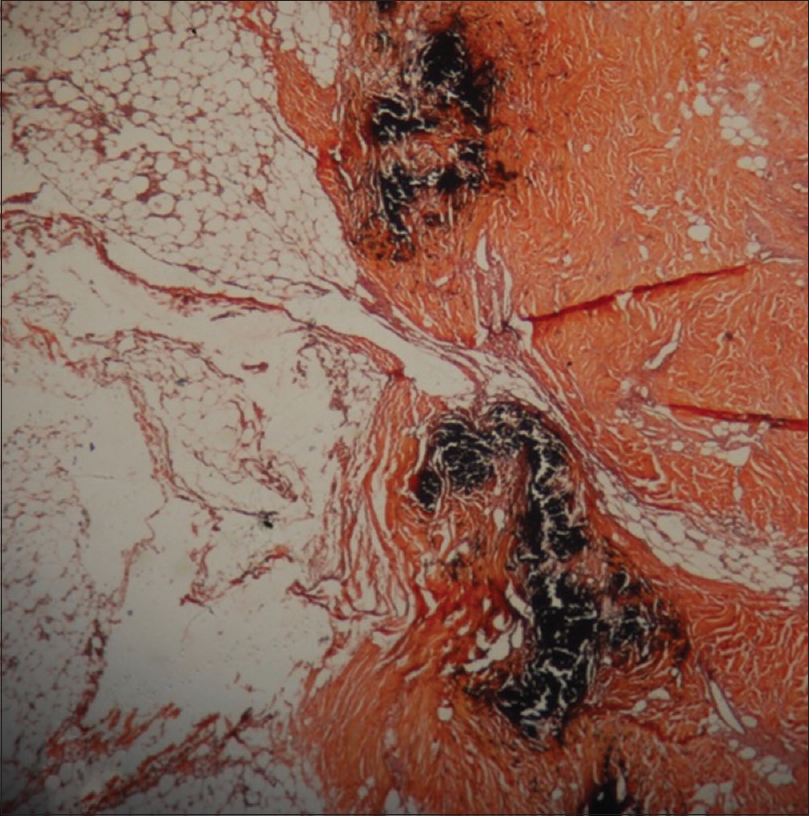 |
| Figure 2: Special stain of lesion demonstrating calcium in the cutaneous arteriole (von Kossa, ×400) |
A diagnosis of calciphylaxis with hyperparathyroidism was made clinically and histopathologically. The patient was given symptomatic and supportive care initially in the form of intravenous fluids, normal saline at the rate of 100 ml/h and intravenous antibiotics – linezolid 600 mg 12 hourly, piperacillin with tazobactam 2.25 g 8 hourly, intravenous pantoprazole 40 mg 12 hourly along with high protein diet and hematinics. However, serum calcium continued to rise (max 15.8 mg/dl) and the patient also developed facial edema. Elective parathyroidectomy was undertaken at this point (2 weeks from admission) to stabilize calcium levels. Intravenous frusemide 40 mg 12 hourly was added. Postoperative period showed a decline in serum calcium levels and increased levels of thyroid stimulating hormone (89.91 IU/ml). Resection of a small portion of thyroid gland was done during parathyroidectomy due to its nonspecific location and this led to iatrogenic hypothyroidism. Thyroxine 100 μg per day was initiated to treat the iatrogenic hypothyroidism. The skin lesions had evolved into hemorrhagic infarcts and necrotic eschar with involvement of bilateral gluteal regions [Figure - 1]b. A wide local excision and wound debridement followed by vacuum-assisted closure dressing was undertaken after 2 weeks. Intravenous antibiotics were changed to meropenem 1 g 12 hourly; and clindamycin 600 mg 12 hourly and intravenous tramadol 50 mg 12 hourly were also added to the treatment regime. One week after wound debridement, hyperbaric oxygen therapy was introduced at 2.5 atmospheric pressure absolute for 90 min, 5 days a week for three consecutive weeks. It led to marked improvement in the size of lesions [Figure - 1]c, pain and general condition of patient along with improvement in lab parameters. Elevated levels of prolactin and cortisol can occur during acute stress response of body and they gradually normalize as the crisis settles, as was seen in this case. Healthy granulation tissue at 4 weeks from initiation of hyperbaric oxygen therapy enabled primary suturing of lesions. The patient is presently on regular follow-up [Figure - 1]d and doing well. Calciphylaxis is described as a systemic medial calcification of the arteries and occurs often in patients with end-stage renal disease or after kidney transplantation. Other risk factors include dialysis, hyperphosphatemia, hypercalcemia, hyperparathyroidism, diabetes mellitus and female gender. However, none of them are individually responsible for causing calciphylaxis.[4]
The pathogenesis of calciphylaxis is poorly understood.[5] It frequently develops in the lower extremities and is often misdiagnosed as vasculitis, diabetic ulceration or cholesterol emboli. Ulceration is rapid and covers large skin areas as in our case, accompanied by impaired wound healing. Histopathologically, there is pathognomonic arteriolar calcification confirmed by staining based on silver nitrate.[3]
Management is challenging and involves multidisciplinary approach with special emphasis on wound care, as was done in our case with the help of vacuum-assisted closure dressing. Negative pressure dressing with surgical excision and conservative treatment have been previously utilized successfully.[6]
Data are also available on the use of agents such as sodium thiosulfate and bisphosphonates in treatment of calciphylaxis.[7],[8] A noncalcium, nonaluminium phosphate binder can be used as an adjunctive therapy. Systemic corticosteroids have also been used, but may cause calcium and phosphorous abnormalities.[9] Cinacalcet is used to lower parathyroid levels as well as improve calcium–phosphorus homeostasis.[10]
We did not use any of these in this case, due to improvement noticed after the surgical excision and the wound care measures in the form of vacuum-assisted closure dressing and hyperbaric oxygen therapy.
Hyperbaric oxygen therapy is a newer therapeutic modality. The patient breathes 100% oxygen at a pressure higher than ambient atmospheric pressure inside a sealed treatment chamber. The possible mechanism of action is that it increases the amount of oxygen in diseased tissue, thereby promoting wound healing. Hyperbaric oxygen at 2.5 atmospheric pressure absolute has been used for 90 min, 5 days per week for 5–7 consecutive weeks with benefit in reported cases of calciphylaxis.[11],[12],[13] The role of parathyroidectomy is considered controversial, but has been found to be helpful in patients with primary hyperparathyroidism [14] as in our case.
The combination of parathyroidectomy with hyperbaric oxygen therapy has been tried to treat calciphylaxis in two cases, however, with an unsuccessful outcome.[15] To conclude, this case of calciphylaxis occurring in a young patient without comorbidity, secondary to parathyroid adenoma, highlights calciphylaxis as its important cutaneous marker and notes its successful management with parathyroidectomy and hyperbaric oxygen therapy.
Acknowledgements
- Anupam Kumar, Associate Professor, Department of Endocrinology, INHS Asvini, Mumbai, Maharashtra, India
- Nilanjan Roy, Associate Professor, Department of Reconstructive Surgery, INHS Asvini, Mumbai, Maharashtra, India
- Aarti Trehan, Associate Professor, Department of Pathology, INHS Asvini, Mumbai, Maharashtra, India.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for the images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Selye H. The dermatologic implications of stress and calciphylaxis. J Invest Dermatol 1962;39:259-75.
[Google Scholar]
|
| 2. |
Dahl PR, Winkelmann RK, Connolly SM. The vascular calcification-cutaneous necrosis syndrome. J Am Acad Dermatol 1995;33:53-8.
[Google Scholar]
|
| 3. |
Ketteler M, Biggar P. Calciphylaxis: Epidemiology, pathophysiology and therapeutic options. BANTAO J 2008;6:1-5.
[Google Scholar]
|
| 4. |
Hussein MR, Ali HO, Abdulwahed SR, Argoby Y, Tobeigei FH. Calciphylaxis cutis: A case report and review of literature. Exp Mol Pathol 2009;86:134-5.
[Google Scholar]
|
| 5. |
Yu Z, Gu L, Pang H, Fang Y, Yan H, Fang W, et al. Sodium thiosulfate: An emerging treatment for calciphylaxis in dialysis patients. Case Rep Nephrol Dial 2015;5:77-82.
[Google Scholar]
|
| 6. |
Maroz N, Mohandes S, Field H, Kabakov Z, Simman R. Calciphylaxis in patients with preserved kidney function. J Am Coll Clin Wound Spec 2014;6:24-8.
[Google Scholar]
|
| 7. |
Velasco N, MacGregor MS, Innes A, MacKay IG. Successful treatment of calciphylaxis with cinacalcet-an alternative to parathyroidectomy? Nephrol Dial Transplant 2006;21:1999-2004.
[Google Scholar]
|
| 8. |
Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 2004;43:1104-8.
[Google Scholar]
|
| 9. |
Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: Natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007;56:569-79.
[Google Scholar]
|
| 10. |
Robinson MR, Augustine JJ, Korman NJ. Cinacalcet for the treatment of calciphylaxis. Arch Dermatol 2007;143:152-4.
[Google Scholar]
|
| 11. |
Basile C, Montanaro A, Masi M, Pati G, De Maio P, Gismondi A, et al. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: A case series. J Nephrol 2002;15:676-80.
[Google Scholar]
|
| 12. |
Podymow T, Wherrett C, Burns KD. Hyperbaric oxygen in the treatment of calciphylaxis: A case series. Nephrol Dial Transplant 2001;16:2176-80.
[Google Scholar]
|
| 13. |
An J, Devaney B, Ooi KY, Ford S, Frawley G, Menahem S, et al. Hyperbaric oxygen in the treatment of calciphylaxis: A case series and literature review. Nephrology (Carlton) 2015;20:444-50.
[Google Scholar]
|
| 14. |
Nigwekar SU. An unusual case of nonhealing leg ulcer in a diabetic patient. South Med J 2007;100:851-2.
[Google Scholar]
|
| 15. |
Malabu UH, Manickam V, Kan G, Doherty SL, Sangla KS. Calcific uremic arteriolopathy on multimodal combination therapy: Still unmet goal. Int J Nephrol 2012;2012:390768.
[Google Scholar]
|
Fulltext Views
3,184
PDF downloads
1,434





