Translate this page into:
Superficial granulomatous pyoderma: A great mimicker
2 Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Muthu Sendhil Kumaran
Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh - 160 012
India
| How to cite this article: Kumar S, Vinay K, Parsad D, Saikia UN, Kumaran MS. Superficial granulomatous pyoderma: A great mimicker. Indian J Dermatol Venereol Leprol 2018;84:374 |
Sir,
Superficial granulomatous pyoderma is a rare variant of pyoderma gangrenosum which usually manifests as a slow, progressive ulcer. Herein, we describe an unusual case of superficial granulomatous pyoderma extensively involving both buttocks with co-existing subcorneal pustular dermatoses in an elderly woman.
A 70-year-old woman presented persistent ulcers on both buttocks and right deltoid region for 6 years. These lesions followed intramuscular analgesic injections received at these sites. Despite extensive tissue involvement, the patient reported only mild discomfort. She had taken multiple courses of oral antibiotics and two courses of anti-tubercular treatment (category I) for 6 months each without any significant response. She had undergone two surgical excisions of the gluteal ulcers but lesions invariably recurred from the margins. She had no known comorbidities and at the time of presentation, she was not on any medication.
Clinical examination revealed multiple, superficial, well-demarcated, ulcero-proliferative plaques of variable size involving the lateral aspect of both gluteal regions which surrounded central cribriform scars, suggesting old, healed ulcers [Figure - 1]a. The base of the ulcer was formed by multiple sieve-like openings with mucopurulent discharge. During the hospital stay, she developed multiple asymptomatic vesiculopustular lesions with hypopyon sign on the lower limbs and trunk [Figure - 1]b. Punch skin biopsy obtained from the ulcer margin showed an ulcerated epidermis with dense acute inflammatory infiltrate in the dermis. Some scattered giant cells were noted forming ill-formed granulomas [Figure - 2]. Stain for acid-fast bacilli and fungus was negative. A biopsy obtained from a vesiculo-pustular lesion showed a sub-corneal cleft with neutrophilic exocytosis into the epidermis. Direct immunofluorescence from the perilesional skin did not reveal tissue-bound immune complexes. Her hematological and biochemical investigations were within normal limits. Serology for anti-nuclear antibody and rheumatoid factor was negative. Serum electrophoresis was negative for M band and Bence-Jones protein was not detected from her urine. Potassium hydroxide preparation and Gram stain were repeatedly negative from the pus. Bacterial, mycobacterial and fungal cultures were all sterile, both from the pus and the skin tissue, on two different occasions. Based on clinical and histological features and repeated negative cultures, a clinical possibility of superficial granulomatous pyoderma was considered and the patient was started on oral prednisolone 1 mg/kg in tapering doses. Lesions showed improvement within a couple of weeks and all active lesions healed completely by 12 weeks with scarring [Figure - 3]. Oral corticosteroids were tapered and stopped by 16 weeks. At 18-month follow-up, the patient continued to be in clinical remission.
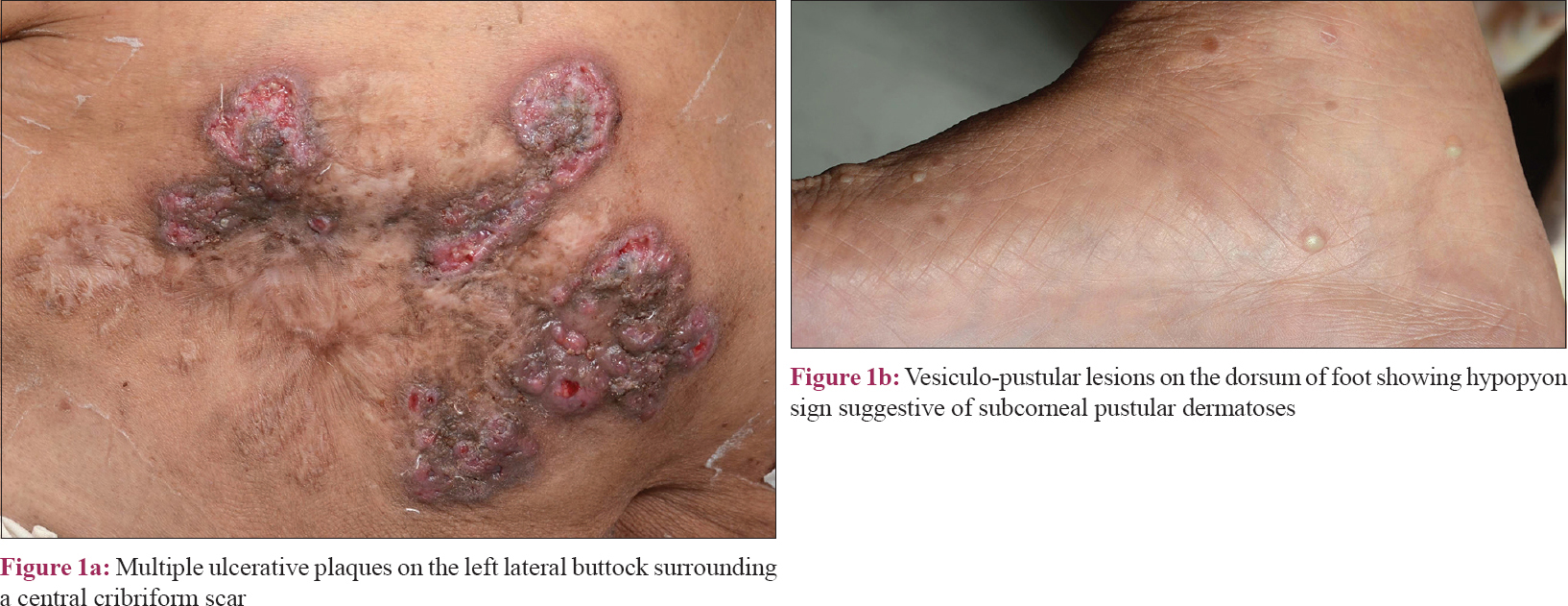 |
| Figure 1 |
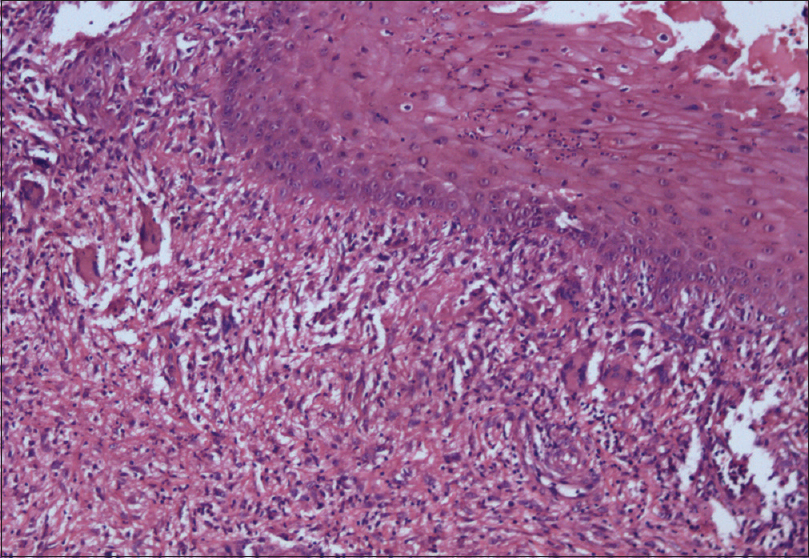 |
| Figure 2: Skin biopsy showing an ulcerated epidermis with dense acute inflammatory infiltrate in the dermis. Scattered giant cells can be appreciated in the upper dermis (H and E, ×200) |
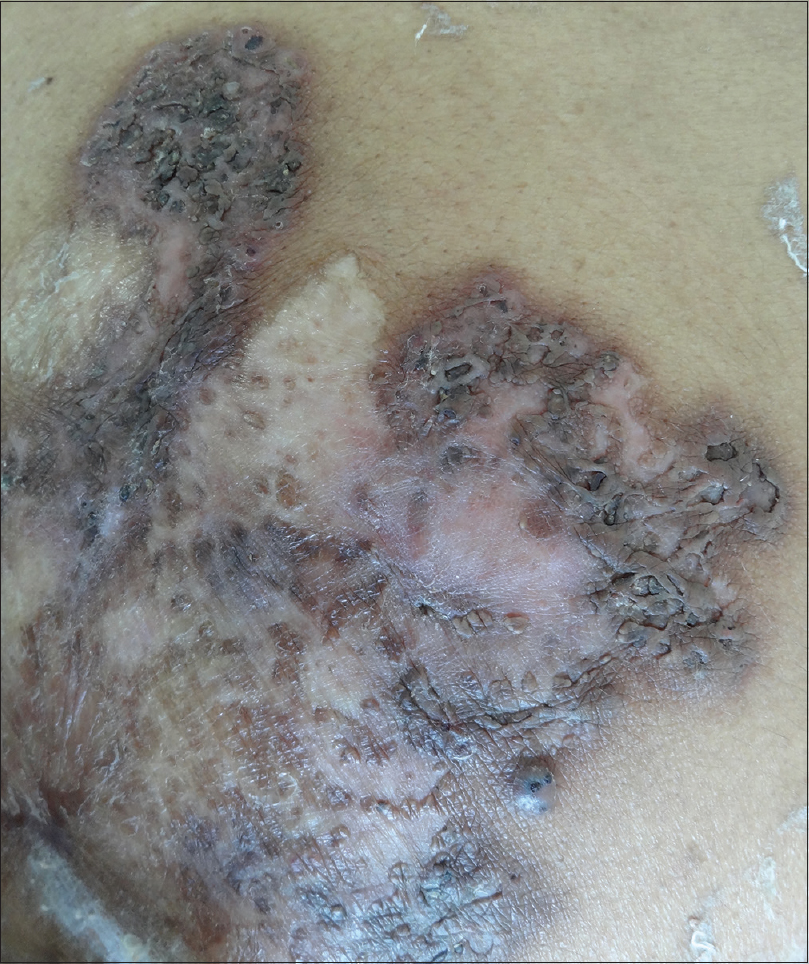 |
| Figure 3: Healing of ulcers with scar formation after 12 weeks of treatment |
Pyoderma gangrenosum is a chronic inflammatory skin disorder whose pathogenesis remains elusive, but immune dysregulation is considered to play a role.[1],[2] Superficial granulomatous pyoderma, initially described by Wilson-Jones and Winkelmann in 1988, differs from classical pyoderma gangrenosum in its clinical presentation, slow progression, granulomatous inflammation, lack of systemic association, good response to steroids and overall, a better prognosis.[3] Clinically, as in our patient, the ulcers in superficial granulomatous pyoderma are multiple, superficial, lack an undermined edge, have a clean base and are almost painless. Histopathologically, they typically show a “three-layer granuloma” in the superficial dermis consisting of central neutrophilic inflammation and necrosis, a surrounding layer of histiocytes and giant cells and an outer layer of plasma cells and eosinophils.[4] Although the characteristic “three-layer granuloma” was not seen in our case, the presence of scattered giant cells and neutrophilic collections was consistent with our clinical diagnosis.
The diagnosis of superficial granulomatous pyoderma is established by the diagnostic criteria proposed by Su et al.[1] The major criteria include the presence of characteristic lesions and exclusion of other causes of ulceration.[1] Minor criteria are characteristic histopathology, lack of systemic associations and rapid response to treatment with steroids. Both the major and at least two of the minor criteria have to be fulfilled for the diagnosis.[1] Our patient satisfied all the above criteria.
Pathergy phenomenon is well described with pyoderma gangrenosum and lesions can develop after surgery or minor trauma.[5],[6] Our patient developed her skin lesions following intramuscular injections and there was recurrence of lesions following surgical excision. Both of these were reminiscent of pathergy. Another interesting clinical clue in our patient was the development of pustular lesions during her inpatient care. Clinical differentials of pustular pyoderma gangrenosum and subcorneal pustular dermatosis were considered. However, the asymptomatic nature of the lesions and hypopyon sign were suggestive of subcorneal pustular dermatosis. Subcorneal pustular dermatoses has been rarely described in association with pyoderma gangrenosum and is considered to be a marker of underlying IgA paraproteinemia.[5] Our patient developed no clinical or laboratory evidence of paraproteinemia during her long follow-up period.
A broad spectrum of treatment modalities has been tried to treat superficial granulomatous pyoderma including glucocorticoids (topical, systemic and intralesional), cyclosporine, tetracyclines, dapsone, clofazamine, sulfapyridine, sulfonamides, thalidomide, tacrolimus (topical and systemic), topical disodium chromoglycate and hyperbaric oxygen. A majority of these patients respond to topical corticosteroids, low-dose systemic corticosteroids or anti-inflammatory agents such as minocycline. In a literature review, it was found that 37% of cases required oral corticosteroids for disease control. However, the dose of systemic corticosteroids required was relatively low when compared to other types of pyoderma gangrenosum.[7] In addition to these agents, wound care and control and treatment of secondary infections are vital.
In conclusion, superficial granulomatous pyoderma is an underdiagnosed entity. There is a lack of awareness among dermatologists about this rare subtype of pyoderma gangrenosum. Despite multiple dermatology consultations, the disease had failed detection for 6 years leading to local destruction and mutilation in our patient. Because of atypical clinical features and presence of granulomatous inflammation on histopathology, it is often misdiagnosed as deep mycosis, atypical mycobacterial infection and lupus vulgaris.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: Clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004;43:790-800.
[Google Scholar]
|
| 2. |
Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: A comprehensive review. Am J Clin Dermatol 2012;13:191-211.
[Google Scholar]
|
| 3. |
Wilson-Jones E, Winkelmann RK. Superficial granulomatous pyoderma: A localized vegetative form of pyoderma gangrenosum. J Am Acad Dermatol 1988;18:511-21.
[Google Scholar]
|
| 4. |
Langan SM, Powell FC. Vegetative pyoderma gangrenosum: A report of two new cases and a review of the literature. Int J Dermatol 2005;44:623-9.
[Google Scholar]
|
| 5. |
Banga F, Schuitemaker N, Meijer P. Pyoderma gangrenosum after caesarean section: A case report. Reprod Health 2006;3:9.
[Google Scholar]
|
| 6. |
Verma SB. Atypical pyoderma gangrenosum following total knee replacement surgery:First report in dermatologic literature. An Bras Dermatol 2009;84:689-91.
[Google Scholar]
|
| 7. |
Stone MS, Lyckholm LJ. Pyoderma gangrenosum and subcorneal pustular dermatosis: Clues to underlying immunoglobulin A myeloma. Am J Med 1996;100:663-4.
[Google Scholar]
|
Fulltext Views
6,606
PDF downloads
2,861





