Translate this page into:
Pigmented linear discoid lupus erythematosus following the lines of Blaschko: A retrospective study of a Chinese series
2 Department of Dermatology, Hospital Ramon y Cajal, Madrid, Spain
Correspondence Address:
Dong-Lai Ma
No. 1 Shuaifuyuan, Dongcheng District, Beijing, 100730
China
| How to cite this article: Liu W, Vano-Galvan S, Liu JW, Qian YT, Fang K, Ma DL. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: A retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol 2020;86:359-365 |
Abstract
Background: Linear cutaneous lupus erythematosus is a rare subtype of lupus erythematosus (LE) that develops linear lesions following the lines of Blaschko. Linear cutaneous lupus erythematosus may present as various subtypes of LE, including linear discoid lupus erythematosus. There are few reports about pigmentedlinear discoid lupus erythematosus in the literature.
Aims: We aimed to summarize the clinical and pathological features of patients with pigmented linear discoid lupus erythematosus following the lines of Blaschko.
Methods: Eighteen patients with pigmented linear discoid lupus erythematosus attending the outpatient department of the Dermatology, Peking Union Medical College Hospital, China, were enrolled in the study. We recorded clinical data including sex, age at onset, disease duration, location and distribution of the lesions, symptoms, trigger factors, antinuclear antibody (ANA) testing, therapy, and therapeutic responses. Histopathological features were also summarized.
Results: All 18 patients presented with well-defined brownish pigmented linear or segmental macules or plaques, following the lines of Blaschko. All the lesions were located on the head or neck. Unilaterally distributed lesions were found in 94.4% of patients. Two patients showed low titers of ANA in a speckled pattern. No systemic involvement or progression to systemic LE was noted. The patients were clinically diagnosed as pigmented lichen planus (55.6%), pigmented linear discoid lupus erythematosus (33.3%), and linear morphea (11.1%) before histopathological examination.
Limitations: The study was retrospective and direct immunofluorescence was not performed. Not all patients' information was available and 4 patients were lost to follow-up because their contact information was changed.
Conclusion: Pigmented linear discoid lupus erythematosus mostly occurs on the head and neck. It manifests as brownish macules along the lines of Blaschko. Differentiation between pigmented linear discoid lupus erythematosus and other dermatoses that have a linear distribution can be difficult both clinically and pathologically, but histological details can help distinguish them.
Introduction
Lupus erythematosus (LE) is an inflammatory autoimmune disease with various clinical presentations. It ranges from the localized form of the disease (restricted to the skin) to the systemic form. Linear cutaneous lupus erythematosus, proposed by Abe et al. in 1998, is a rare subtype of LE that presents with linear lesions.[1] The lesions are usually limited to the skin without systemic involvement, and progression to SLE is infrequent.[2] About 100 cases of linear cutaneous lupus erythematosus have been reported in literature,[2] and it may present as linear discoid lupus erythematosus, LE profundus, subacute cutaneous lupus erythematosus (SCLE), bullous LE, and linear localized scleroderma associated with discoid lupus erythematosus (DLE).[3],[4],[5],[6],[7],[8] DLE is characterized by erythematous, atrophic, hyperkeratotic, dyschromic discoid lesions, and linear discoid lupus erythematosus develops with a linear or segmental arrangement of DLE lesions following the lines of Blaschko. There have been a few case reports about pigmented linear discoid lupus erythematosus, which can be confusing in clinical differentiation from pigmented lichen planus, lichen planopilaris, atypical psoriasis, lichen sclerosus et atrophicus, lichen striatus and other dermatoses.[9],[10]
The objective of this study was to describe a series of 18 Chinese patients with pigmented linear discoid lupus erythematosus following the lines of Blaschko and to characterize the clinical and histopathological features in them.
Methods
A key word “linear lupus erythematosus” was searched in our electronic medical system, and cases with pigmentation lesion records were collected. Finally, there were 18 pigmented linear discoid lupus erythematosus patients with comprehensive available information seen at our department of Dermatology between 2006 and 2017. The diagnosis of pigmented linear discoid lupus erythematosus had been previously made with clinical photographs, medical records, and histopathological confirmation. For all patients, we obtained the clinical data from the records including sex, age at onset, disease duration, location and distribution of the lesions, symptoms, trigger factors, antinuclear antibody (ANA) testing, therapy and therapeutic response. Formalin-fixed and paraffin-embedded skin biopsy specimens from all patients were re-examined by two dermatopathologists, and histopathological features were recorded
Results
Demographic and clinical data of the patients are presented in [Table - 1]. There was a total of 18 patients including 10 male patients and 8 female patients, and the male-to-female ratio was 1.25:1. The mean age at onset was 39.1 years (range 12–60 years). The mean duration of lesions at presentation was 9.7 months (range 10 days–3 years).
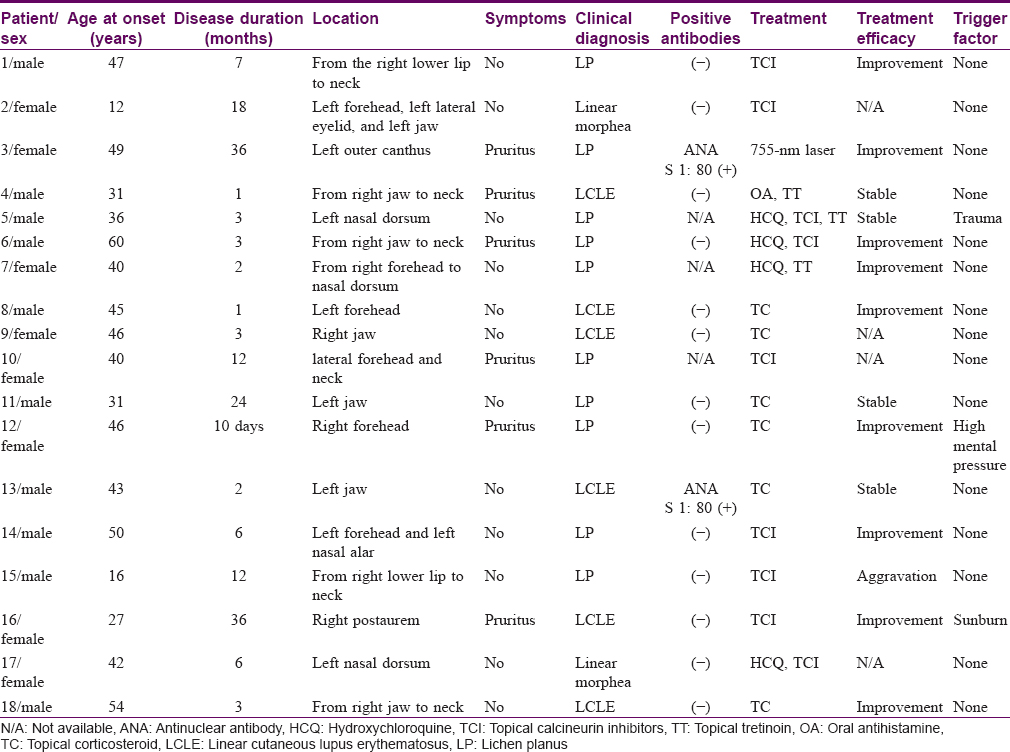
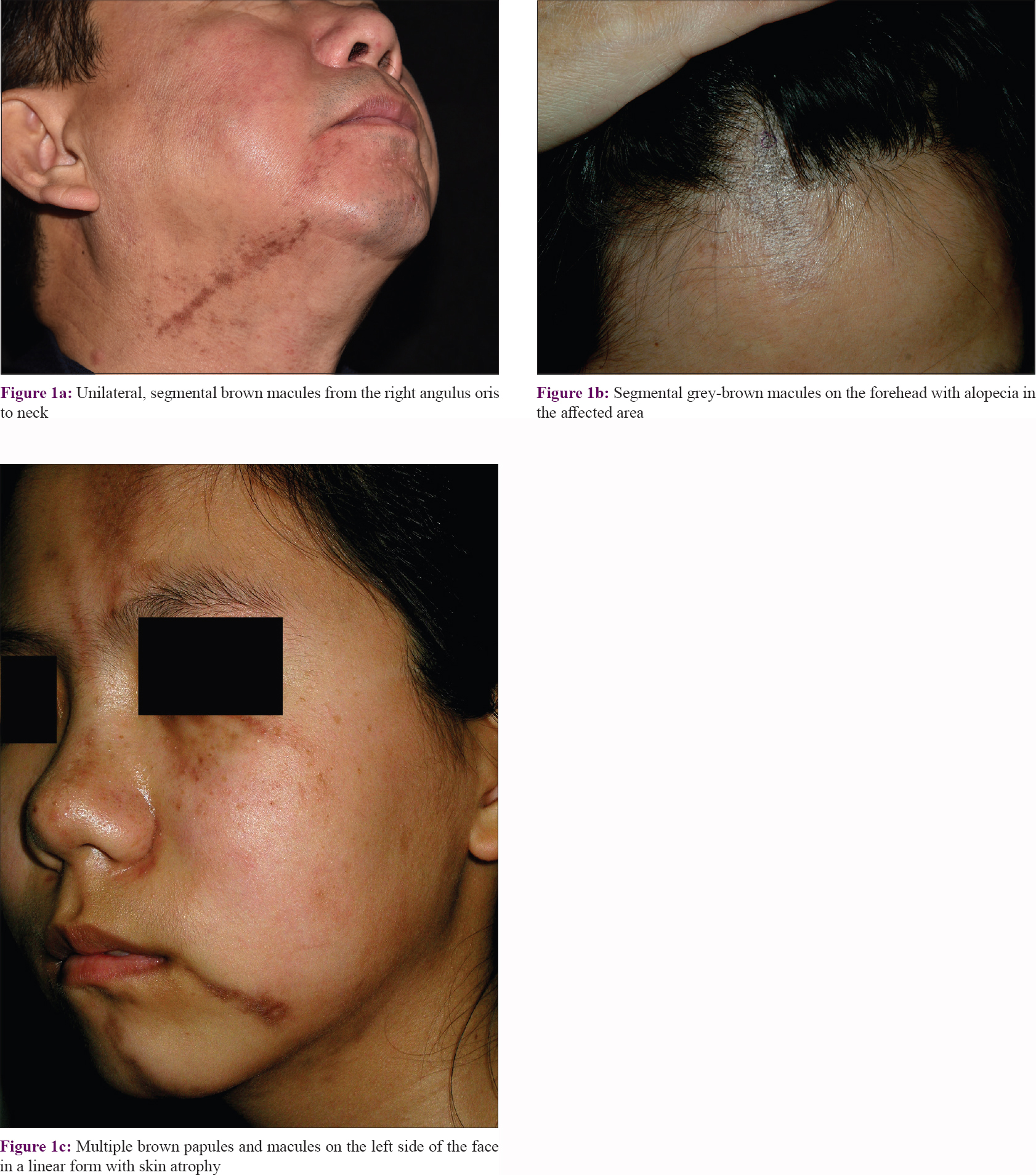
All 18 patients presented with well-defined brownish pigmented linear or segmental macules or plaques [Figure - 1] following the lines of Blaschko, and 14 patients presented with atrophic lesions. All the lesions were located on the head or neck. Lesions were unilateral in 17 (94.4%) patients with 9 cases on the right side and 8 cases on the left, whereas 1 (5.6%) patient had bilateral involvement. Most lesions were asymptomatic, and only six patients had slightly itching. Three patients recalled possible trigger factors: one patient had preceding local injury 3 months before appearance of the lesion, one patient suffered from high mental stress at work, and one patient had a sunburn before the lesions appeared. Alopecia of the involved area was noted in one patient. There was no history of similar cases in the family or history of photosensitivity in any of the 18 patients. No systemic involvement was noted in the record available. Two patients showed low titers of ANA, in a speckled pattern. Dermoscopic examination was performed in one patient and showed blue-whitish pigment granules, reticulate pigmentation, and white structureless areas [Figure - 2]. Follow-up data were available for 14 patients [Table - 1].
 |
| Figure 1: |
 |
| Figure 2: |
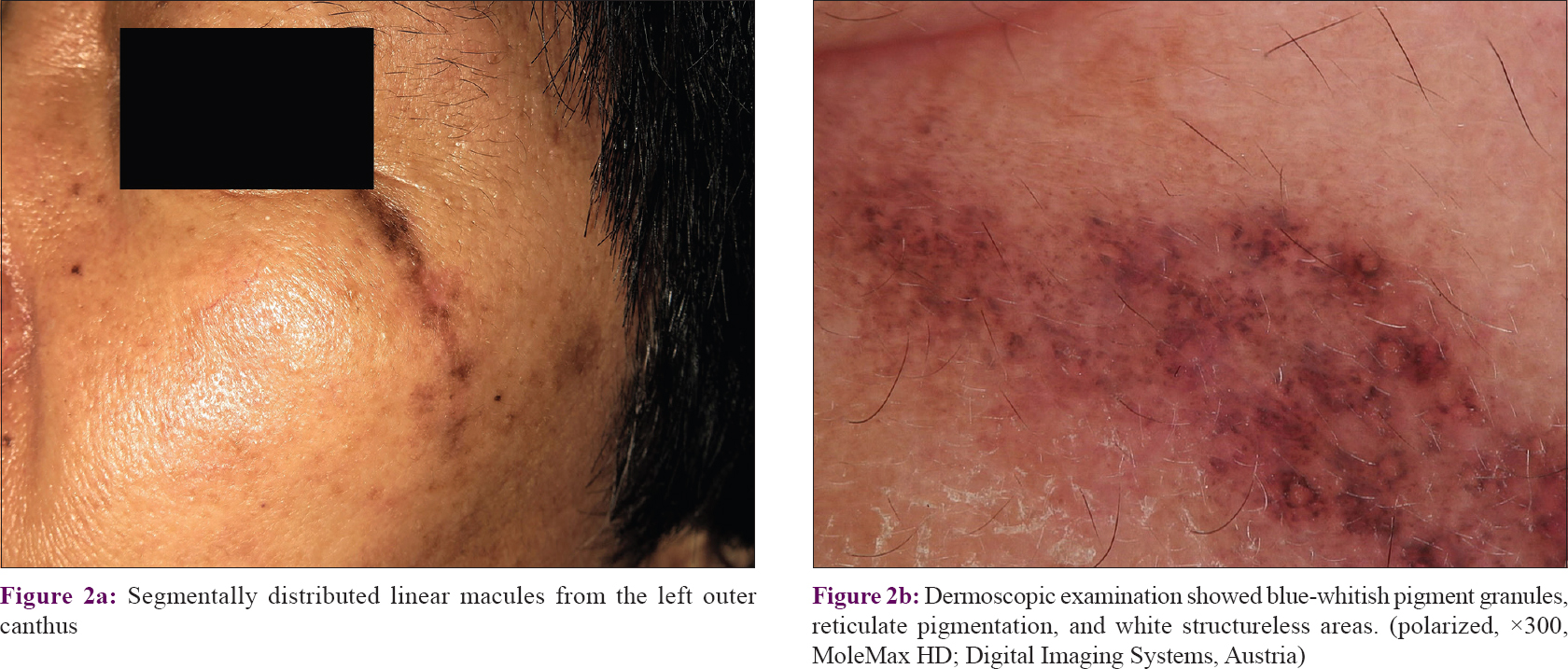
Regarding therapy, the patients had received various treatment options, such as topical calcineurin inhibitor (n = 9; 50%), oral antihistamine (n = 1; 5.6%), hydroxychloroquine (n = 4; 22.2%), topical corticosteroid (n = 6; 33.3%), topical tretinoin (n = 3; 16.7%), and 755-nm laser (n = 1; 5.6%). Lesions improved in nine patients, remained stable in four patients, worsened in one patient, and four patients were lost to follow-up. No progression to SLE was noted within a median follow-up of 2.2 years duration (ranging from 2 months to 9 years). Patients were clinically diagnosed as pigmented lichen planus (n = 10; 55.6%), pigmented linear discoid lupus erythematosus (n = 6; 33.3%), and linear morphea (n = 2; 11.1%) before the histopathological examination. The diagnosis of pigmented linear discoid lupus erythematosus was confirmed by histopathological findings in all 18 patients, with hyperkeratosis, follicular plugging, epidermal atrophy, vacuolar basal cell degeneration, pigmentary incontinence in the upper dermis, and perivascular and periadnexal infiltration [Figure - 3]. Obvious hair follicle destruction and reduction in number of follicles were noted in four patients. Periodic acid–Schiff (PAS) staining showed basal membrane thickening and a mucin stain revealed mucin deposition in the dermis [Figure - 4]. Direct immunofluorescence (DIF) testing was not done since it is not performed routinely in our department.
 |
| Figure 3: |
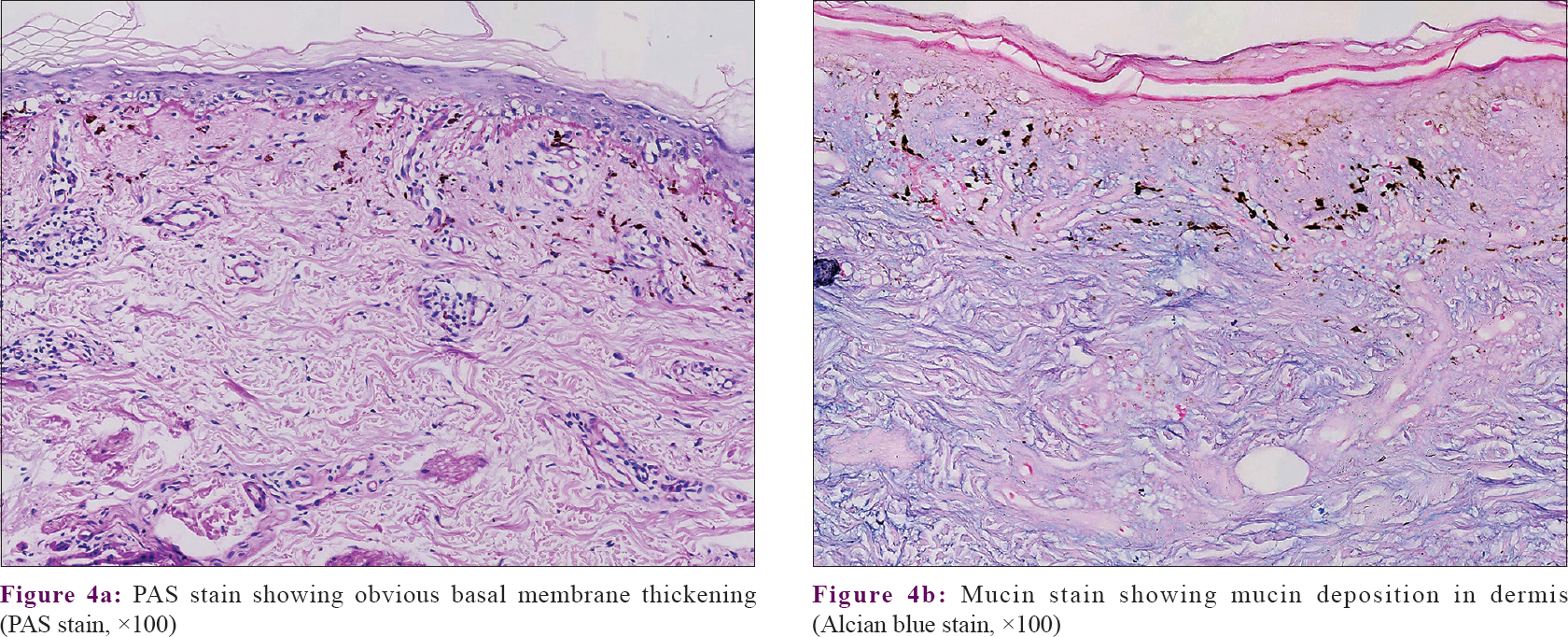 |
| Figure 4: |
Discussion
The linear and segmental manifestations of DLE along the lines of Blaschko suggest a different pathomechanism of DLE in these cases. It is widely accepted that cutaneous lesions following Blaschko lines are caused by genetic mosaicism, reflecting the pathways of migration and proliferation of abnormal keratinocytes arising during embryogenesis.[11],[12] The cells arising from these mosaicisms express neoantigens capable of eliciting local immune responses.[4] Therefore, linear discoid lupus erythematosus along the lines of Blaschko may related to the lesional genetically abnormal skin cells rather than the normal immune cells.[13] Nearly half of the patients with linear LE were children younger than 18 years old in one report.[2] But the existence of patients with a later onset indicates that there may be other factors besides genetic predisposition that affect the appearance of skin lesions.[2] The trigger for the onset of the cutaneous disease may be trauma, viral infection, primary irritation, or exogenous agents such as ultraviolet light, drugs, pesticides, heavy metals, or other elements.[14],[15],[16] This may explain why 11 patients (61.1%) in our series had a late onset of the disease (≥40 years).
To summarize the literature, the lesions of pigmented linear cutaneous lupus erythematosus most commonly develop on the head (68.8%), followed by the upper limbs, trunk, neck and lower limbs.[2] All the lesions of pigmented linear discoid lupus erythematosus in our series were located on the head and neck, and no limb or trunk involvement was found. In the majority of our cases, the lesions were restricted unilaterally to one anatomical site, whereas there have also been reports of bilateral or widespread lesions.[17],[18] Predominance in females is also described in the literature,[2] but there is a slight male predilection (M/F: 10/8) in our case series, which may be explained by a small sample size. Photosensitivity may be observed, though all our cases denied having photosensitivity.
Linear discoid lupus erythematosus has been usually characterized as comprising scaly, papular or erythematosus lesions with partly whitish atrophy arranged in linear or segmental patterns. Calcinosis cutis and secondary milia are uncommon manifestations.[19] Scalp involvement can cause scarring alopecia. In contrast, pigmented linear discoid lupus erythematosus in our series presented as brownish continuous macules following the lines of Blaschko, with or without atrophy, and no discoid or scaly lesions were noted. The lesions can be asymptomatic or pruritic.[2] Most commonly, ANA are negative or with low positive titers.
The histological characteristics of pigmented linear discoid lupus erythematosus include hyperkeratosis, follicular plugging, atrophy of the epidermis and liquefaction degeneration of the basal layer, with dense perivascular and periadnexal lymphocytic infiltrates and pigmentary incontinence in the dermis. Civatte bodies may present in some cases.[9] PAS staining reveals thickening of the epidermal basement membrane, and alcian blue staining reveals mucin deposition in the reticular dermis. DIF of lesional skin is positive in most cases.[9],[20]
Pigmented linear discoid lupus erythematosus did not clinically present as discoid, erythematosus, and scaly lesions in our series and only six of our patients (33.3%) were correctly diagnosed before histopathological examination. Several inflammatory dermatoses follow the lines of Blaschko or present linear configurations including linear discoid lupus erythematosus, psoriasis, linear morphea, lichen sclerosus et atrophicus, lichen striatus, and especially linear lichen planus and linear lichen planopilaris are important differential diagnosis. Linear lichen planus is the most important differential diagnosis of pigmented linear discoid lupus erythematosus, because they both can manifest as linear pigmented or nonpigmented macules with liquefaction degeneration of the basal layer and dense lymphocytic infiltrates on histopathology. But classic histopathologic findings such as necrotic keratinocytes and a lichenoid infiltrate in the papillary dermis, together with the absence of dilated infundibula with plugs of cornified cells, thickened basement membrane, perivascular and periadnexal infiltrates and mucin deposition can point to a diagnosis of lichen planus.[21],[22] Lichen planopilaris is considered as a follicular variant of lichen planus. It typically distributed over the scalp, although nonscalp areas can rarely be affected. However, most of the patients in our series had lesions distributed over the face and neck, and only six patients had partial scalp involvement. It is reported that more than 50% of patients with lichen planopilaris have scalp pruritus, perifollicular erythema, and perifollicular scaling at presentation,[23] which were absent in all 18 patients. Histopathologically, inflammation affects the hair follicles with perifollicular fibrosis, and decreased numbers of hair follicles can be seen in different stages of lichen planopilaris. While thickening of the epidermal basement membrane and mucin deposition are also absent in lichen planopilaris. Lichen striatus rarely occurs on the face, and the epidermal basement membrane thickening, dermal deposition of mucin, and positive finding on direct immunofluorescence are present in LE but not in lichen striatus. Histopathological manifestations such as confluent parakeratosis in the stratum corneum, regular club-shaped acanthosis of the epidermis, and dilated blood vessels in dermal papillae are compatible with psoriasis. Thick, closely packed, hyalinized collagen bundles in the dermis help differentiate linear morphea from linear discoid lupus erythematosus. The distinctive thinned epidermis and papillary dermal homogenization in lichen sclerosus et atrophicus can help rule out linear discoid lupus erythematosus. Other diseases that have perivascular and periadnexal infiltrates in the dermis such as leprosy, syphilis, polymorphous light eruption and arthropod bites should also be considered as pathologic differential diagnosis.
Treatment of pigmented linear discoid lupus erythematosus may be challenging. For patients with photosensitivity, strict sun protection is suggested. Topical corticosteroid and topical calcineurin inhibitors are topical treatment options for cases presenting with limited cutaneous lesions. Physical treatments such as laser therapy, cryotherapy, and dermabrasion can also be selected.[19] Other systemic therapies may be used in recalcitrant cases, such as antimalarial drugs, corticosteroids, immunosuppressive drugs, biological drugs, dapsone, thalidomide and oral retinoids.[2],[17],[19]
The limitations of our study include that this is a retrospective investigation in one medical center relying on the review of electronic data. It should be noted that there maybe some cases missed since we only searched the key word “linear lupus erythematosus” in our electronic medical system and some cases may be misdiagnosed to other dermatoses and were not recorded under this index. We did not see all patients in person to systematically categorize skin findings and the results may not be generalizable. And direct immunofluorescence was not performed.
Conclusion
We present a series of 18 Chinese patients with pigmented linear discoid lupus erythematosus. Clinically, pigmented linear discoid lupus erythematosus manifests as brownish macules along the lines of Blaschko. The differentiation between pigmented linear discoid lupus erythematosus and other linear dermatoses such as pigmented linear lichen planus and linear lichen planopilaris can be difficult clinically, but histological details can help distinguish these different entities. The recognition of such rare DLE subtype may have important clinical implications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Abe M, Ishikawa O, Miyachi Y. Linear cutaneous lupus erythematosus following the lines of blaschko. Br J Dermatol 1998;139:307-10.
[Google Scholar]
|
| 2. |
Jin H, Zhang G, Zhou Y, Chang C, Lu Q. Old lines tell new tales: Blaschko linear lupus erythematosis. Autoimmun Rev 2016;15:291-306.
[Google Scholar]
|
| 3. |
Bouzit N, Grézard P, Wolf F, Balme B, Perrot H. Linear cutaneous lupus erythematosus in an adult. Dermatology 1999;199:60-2.
[Google Scholar]
|
| 4. |
Julià M, Mascaró JM Jr., Guilabert A, Navarra E, Ferrando J, Herrero C. Sclerodermiform linear lupus erythematosus: A distinct entity or coexistence of two autoimmune diseases? J Am Acad Dermatol 2008;58:665-7.
[Google Scholar]
|
| 5. |
Elbendary A, Griffin J, Li S, Tlougan B, Junkins-Hopkins JM. Linear sclerodermoid lupus erythematosus profundus in a child. Am J Dermatopathol 2016;38:904-9.
[Google Scholar]
|
| 6. |
Mao QX, Zhang WL, Wang Q, Xiao XM, Chen H, Shao XB, et al. Linear cutaneous lupus erythematosus/discoid lupus erythematosus in an adult. Postepy Dermatol Alergol 2017;34:177-9.
[Google Scholar]
|
| 7. |
Frances L, Betlloch I, Leiva-Salinas M, Marin I, Pascual JC. Subacute cutaneous lupus erythematosus starting as linear lupus erythematosus. Int J Dermatol 2016;55:173-6.
[Google Scholar]
|
| 8. |
Roholt NS, Lapiere JC, Wang JI, Bernstein LJ, Woodley DT, Eramo LR. Localized linear bullous eruption of systemic lupus erythematosus in a child. Pediatr Dermatol 1995;12:138-44.
[Google Scholar]
|
| 9. |
Thind CK, Husain EA, Hewitt J. A rare linear atrophic lesion on the face. Clin Exp Dermatol 2009;34:e447-8.
[Google Scholar]
|
| 10. |
Kim J, Lee SH, Roh MR. Linear cutaneous lupus erythematosus on the midline of the face. J Dermatol 2011;38:609-12.
[Google Scholar]
|
| 11. |
Bolognia JL, Orlow SJ, Glick SA. Lines of blaschko. J Am Acad Dermatol 1994;31:157-90.
[Google Scholar]
|
| 12. |
Happle R. Mosaicism in human skin. Understanding the patterns and mechanisms. Arch Dermatol 1993;129:1460-70.
[Google Scholar]
|
| 13. |
Kawachi Y, Taguchi S, Fujisawa Y, Furuta J, Nakamura Y, Ishii Y, et al. Linear childhood discoid lupus erythematosus following the lines of blaschko: Successfully treated with topical tacrolimus. Pediatr Dermatol 2011;28:205-7.
[Google Scholar]
|
| 14. |
Shapiro M, Sosis AC, Junkins-Hopkins JM, Werth VP. Lupus erythematosus induced by medications, ultraviolet radiation, and other exogenous agents: A review, with special focus on the development of subacute cutaneous lupus erythematosus in a genetically predisposed individual. Int J Dermatol 2004;43:87-94.
[Google Scholar]
|
| 15. |
Aiyama A, Muro Y, Sugiura K, Onouchi H, Akiyama M. Extraordinarily long linear cutaneous lupus erythematosus along the lines of blaschko. Dermatol Online J 2013;19:18960.
[Google Scholar]
|
| 16. |
Zandman-Goddard G, Solomon M, Rosman Z, Peeva E, Shoenfeld Y. Environment and lupus-related diseases. Lupus 2012;21:241-50.
[Google Scholar]
|
| 17. |
Verma SB, Wollina U. Chronic disseminated discoid lupus erythematosus with linear lesions following blaschko's lines on forearm and hand. J Dtsch Dermatol Ges 2012;10:129-30.
[Google Scholar]
|
| 18. |
Sàbat M, Ribera M, Bielsa I, Mangas C, Fernández-Chico N, Ferrándiz C. Linear lupus erythematosus following the lines of blaschko. J Eur Acad Dermatol Venereol 2006;20:1005-6.
[Google Scholar]
|
| 19. |
Ma H, Liao M, Qiu S, Lu R, Lu C. Linear cutaneous lupus erythematosus with calcinosis cutis and milia. Pediatr Dermatol 2015;32:e33-5.
[Google Scholar]
|
| 20. |
Alcántara-González J, Fernandez-Guarino M, Carrillo-Gijon R, Jaén-Olasolo P. Linear cutaneous lupus erythematosus. Indian J Dermatol Venereol Leprol 2011;77:717-9.
[Google Scholar]
|
| 21. |
Requena C, Torrelo A, de Prada I, Zambrano A. Linear childhood cutaneous lupus erythematosus following blaschko lines. J Eur Acad Dermatol Venereol 2002;16:618-20.
[Google Scholar]
|
| 22. |
Marinho AK, Ramos TB, Barbosa DM, Kozmhinsky V, Takano DM, Figueira MM. Linear cutaneous lupus erythematosus following the lines of blaschko – Case report. An Bras Dermatol 2016;91:510-3.
[Google Scholar]
|
| 23. |
Kerkemeyer KL, Green J. Lichen planopilaris: A retrospective study of 32 cases in an Australian tertiary referral hair clinic. Australas J Dermatol 2018;59:297-301.
[Google Scholar]
|
Fulltext Views
5,439
PDF downloads
1,723





