Translate this page into:
Topical photodynamic therapy in the treatment of basal cell carcinoma in Singaporean Chinese patients
2 Department of Dermatology, Skin and Cancer Foundation, Darlinghurst, Sydney, Australia
Correspondence Address:
Hui-Yi Chia
National Skin Centre, 1 Mandalay Road Singapore 308205
Singapore
| How to cite this article: Chia HY, Koh SLC, Theng TSC, Chong WS. Topical photodynamic therapy in the treatment of basal cell carcinoma in Singaporean Chinese patients. Indian J Dermatol Venereol Leprol 2015;81:151-154 |
Abstract
Background: Topical photodynamic therapy has been used for the treatment of superficial and nodular basal cell carcinomas, with varying cure rates. Aims: This study aims to evaluate the effectiveness of topical photodynamic therapy in the treatment of superficial and nodular basal cell carcinomas in Asian patients treated at the National Skin Centre, Singapore. Materials and Methods: A retrospective analysis of Asian patients with histologically confirmed basal cell carcinomas and treated with photodynamic therapy was performed. Results: Eight Chinese patients, with an equal gender distribution and mean age of 83.4 years were included. Five of eight basal cell carcinomas were superficial while the remaining three were nodular. The basal cell carcinomas were located in the head and neck in seven patients. The overall clearance rate at 3 months was 87.5% while the clearance rate for superficial and nodular basal cell carcinomas was 100% and 66.6% respectively at 3 months. At 12 months, the overall clearance rate was 85. 7%. Limitations: This is a retrospective analysis with small patient numbers. Conclusions: In this small series of eight Asian patients, topical photodynamic therapy has been shown to be effective and generally well-tolerated in the treatment of basal cell carcinomas, particularly of the superficial subtype. However, larger studies are needed to evaluate its overall efficacy in Asian patients.INTRODUCTION
The most commonly encountered skin cancer is the basal cell carcinoma (BCC). They frequently occur on sun-exposed sites such as the face, neck, upper extremities and trunk, and are typically slow-growing, locally invasive and rarely metastasize. Factors to be considered before determining the most appropriate treatment modality for an individual patient include the histologic subtype, its size and location, and whether any concommitant, relevant medical issues are present. The current gold standard of treatment is surgical excision. Alternative treatment options include curettage and cautery, cryotherapy, radiation therapy, immune response modifiers and more recently topical photodynamic therapy (PDT). [1]
Photodynamic therapy involves the activation of a photosensitizer, usually a porphyrin derivative by irradiation from an appropriate light source. Topical 5-aminolevulinic acid (ALA) or methyl aminolevulinate (MAL) are commonly used topical photosensitizers. This therapy is highly effective in the treatment of superficial basal cell carcinoma with reported cure rates of 87-100%, but lower cure rates (10-76%) have been reported in the treatment of nodular basal cell carcinoma. [2],[3],[4],[5],[6]
Materials and Methods
Patients
A retrospective analysis of consecutive Asian patients (Fitzpatrick Skin Type IV) with histologically confirmed basal cell carcinoma, seen at the National Skin Centre from March 1, 2006 to December 31, 2010 was performed. The study was approved by the national ethics committee.
Treatment protocol
A 1-mm thick layer of methyl aminolevulinate (MAL) cream (Metvix 160 mg/g; Photocure ASA, Hoffsveien 48, NO-0377, Oslo, Norway) was applied to each lesion and subsequently covered with an occlusive dressing for 3 h. The dressings were removed after 3 h and the cream washed off with 0.9% saline solution before illumination with red light using a light-emitting diode lamp (Aktilite CL128, PhotoCure ASA, Hoffsveien 48, NO-0377, Oslo, Norway) at 570-670 nm (peak wavelength at 630 nm). The total irradiation dose was 37 J/cm 2 at 5-8 cm lamp-skin distance, with a lamp irradiance of 75 mW/cm 2 . This process was then repeated 1 week later.
All patients underwent the same treatment protocol except for one patient with a pigmented nodular basal cell carcinoma on the scalp. This patient received a higher first irradiation dose (44 J/cm 2 ) and 2 further sessions at the usual irradiation dose of 37 J/cm 2 . She received two treatments 1 week apart and the third at 1 month owing to persistence of the basal cell carcinoma.
Data Analysis
Patients were reviewed clinically for response at 1, 3, 6 and 12 months after the last therapy session. A visual analogue pain scale (VAS) (score 0 to 10 where 0 represented no pain and 10 the highest pain level) was used to score the intensity of pain experienced during and after photodynamic therapy. Any local or systemic adverse events were recorded during the treatment and subsequently at each follow-up visit.
RESULTS
Baseline clinical characteristics
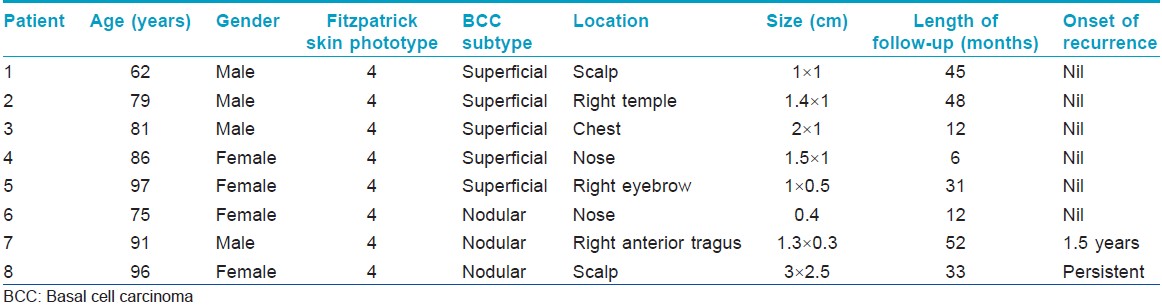
Eight Chinese patients with histologically confirmed basal cell carcinoma were treated with methyl aminolevulinate-photodynamic therapy (MAL-PDT) at the National Skin Centre, Singapore from March 2006 to December 2010. All patients were of Fitzpatrick skin phototype IV. There were 4 males and 4 females, with a mean age of 83.4 years (range 62-97 years). Each patient had a single basal cell carcinoma. Five of eights basal cell carcinoma (62.5%) were superficial while the remaining three (37.5%) were nodular. The lesions were located in the head and neck in seven (62.5%) patients and on the chest in one patient. The size ranged from 0.4 to 3 cm [Table - 1].
Response rates at 3, 6 and 12 months
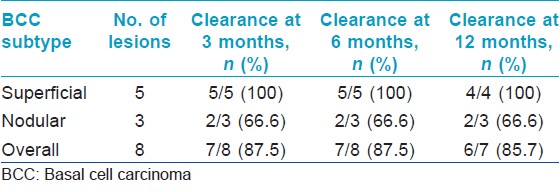
Follow-up data was available for all eight patients at 3 and 6 months, and for seven patients at 12 months. The overall clearance rate for all basal cell carcinomas at 3 months was 87.5% (7 out of 8 patients) while the clearance rate for superficial and nodular basal cell carcinomas was 100% (5 out of 5 patients), and 66.6% (2 out of 3 patients) respectively at 3 months. At 12 months, the overall clearance rate was 85.7% (6 out of 7 patients) [Table - 2] and [Figure - 1]. The mean duration of follow-up was 30.1 (range 6 to 52) months. One patient had a persistent nodular basal cell carcinoma while another had a recurrence of the nodular basal cell carcinoma at 18 months.
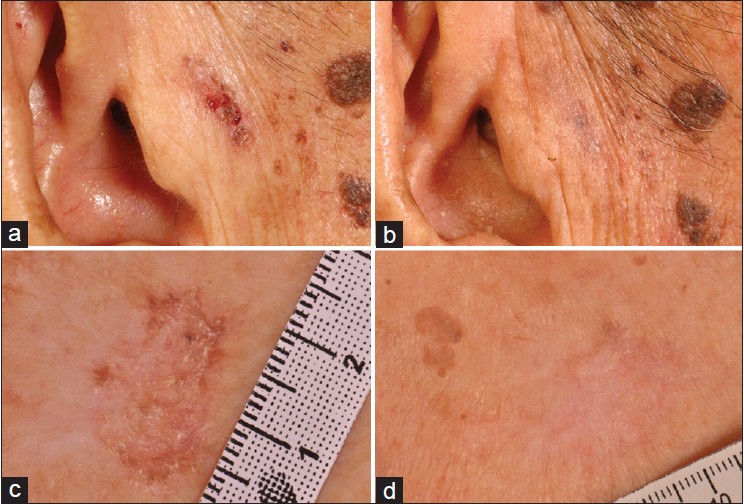 |
| Figure 1: Pigmented nodular BCC at the pre-auricular region before (a) and with clearance (b) at 1 month post-PDT, and superficial BCC at the chest before (c) and with clearance (d) at 3 months post-PDT |
Safety and tolerability
The main side effects experienced were mild-to-moderate erythema, pain and edema during light application. Pain was the most common side effect. The mean recorded visual analogue score (VAS) pain score was 4.5 (range 2-8) among seven patients. The pain score was not available for one patient.
DISCUSSION
The 2008 British guidelines for the management of basal cell carcinoma currently recommends photodynamic therapy as a good treatment for primary superficial basal cell carcinoma with a strength of recommendation A and quality of evidence I, and as a reasonable treatment for primary low-risk nodular basal cell carcinoma, with a strength of recommendation B and quality of evidence I. [1] Photodynamic therapy is not recommended for high-risk basal cell carcinomas.
There is limited published data on the use of topical photodynamic therapy in the treatment of basal cell carcinoma in Asian skin. Li et al. [7] assessed 95 basal cell carcinomas in 47 patients with skin type IV/V with a complete response rate of 77.9% at 12 months. The complete response rates of superficial and nodular basal cell carcinomas in their study at 12 months were 94.9% and 78.6% respectively. In our study, the overall clearance rate for all basal cell carcinomas at 3 and 12 months was 87.5% and 85.7% respectively, which is comparable to earlier published studies. The patient who did not achieve clinical clearance was an elderly 96-year-old female with a pigmented nodular basal cell carcinoma on her scalp who had declined surgery and opted for a trial of photodynamic therapy instead. A higher initial irradiation dose of 44 J/cm 2 was given as pigmented basal cell carcinomas do not allow an optimal penetration of light. A hurdle in the use of this treatment for treatment of basal cell carcinomas in Asian patients is the photoprotective effect of melanin. Melanin absorbs light over the wavelength used for photodynamic therapy (630 nm), which results in inadequate penetration of light into the tumor base [8] and it also can reduce singlet oxygen yields and scavenge free radical species. [9] Radical scavenging limits the efficacy of ionizing radiation. [10] Eumelanin absorbs light between 300 and 1000 nm. At 630 nm, it absorbs 10-15% of incident light. The manufacturer of Aktilite TM suggests increasing the doses of red light from 37 to 41-43 J/cm 2 to compensate for melanin absorption.
Treatment was generally well tolerated in our patients. The main adverse event associated with photodynamic therapy is pain. Patients usually describe a burning or stinging sensation during illumination, usually resolving within hours but sometimes persisting for several days. Pain relief for the majority of patients involves cooling the illumination site with cold water. Local anesthesia may be given to patients experiencing severe pain. None of the patients were documented to have hyperpigmentation after therapy but one patient was noted to have postinflammatory depigmentation. Photodynamic therapy is expensive and this may be a deterrent for more widespread use of this modality in patients with superficial basal cell carcinoma.
There are several limitations in our study including the fact that this was a retrospective analysis with small patient numbers. Also, data on tumor depth were not available, and tumor clearance was not confirmed histologically.
Phtodynamic therapy appears to be a useful treatment modality for superficial basal cell carcinoma in Asian patients and is generally well-tolerated. Further large studies are needed to evaluate its overall efficacy in Asian patients.
| 1. |
Telfer NR, Colver GB, Morton CA. British Association of Dermatologists. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008;159:35-48.
[Google Scholar]
|
| 2. |
Rhodes LE, de Rie MA, Leifsdottir R, Yu RC, Bachmann I, Goulden V, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol 2007;143:1131-6.
[Google Scholar]
|
| 3. |
Calzavara-Pinton PG. Repetitive photodynamic therapy with topical delta-aminolaevulinic acid as an appropriate approach to the routine treatment of superficial non-melanoma skin tumours. J Photochem Photobiol B 1995;29:53-7.
[Google Scholar]
|
| 4. |
Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, et al. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997;79:2282-308
[Google Scholar]
|
| 5. |
Soler AM, Warloe T, Berner A, Giercksky KE. A follow-up study of recurrence and cosmesis in completely responding superficial and nodular basal cell carcinomas treated with methyl 5-aminolaevulinate-based photodynamic therapy alone and with prior curettage. Br J Dermatol 2001;145:467-71.
[Google Scholar]
|
| 6. |
Surrenti T, De Angelis L, Di Cesare A, Fargnoli MC, Peris K. Efficacy of photodynamic therapy with methyl aminolevulinate in the treatment of superficial and nodular basal cell carcinoma: An open-label trial. Eur J Dermatol 2007;17:412-5.
[Google Scholar]
|
| 7. |
Li Q, Gao T, Jiao B, Hu X, Luan Q, Li K, et al. Tumor Thickness Predicts Long-Term Complete Response of Facial Basal Cell Carcinomas in Asian Skin Types IV/V Treated with Methyl Aminolaevulinate Photodynamic Therapy. Photomed Laser Surg 2011;29:501-7.
[Google Scholar]
|
| 8. |
Pass HI. Photodynamic therapy in oncology: Mechanisms and clinical use. J Natl Cancer Inst 1993;85:443-56.
[Google Scholar]
|
| 9. |
Hadjur C, Richard MJ, Parat MO, Jardon P, Favier A. Photodynamic effects of hypericin on lipid peroxidation and antioxidant status in melanoma cells. Photochem Photobiol 1996;64:375-81.
[Google Scholar]
|
| 10. |
Slominski A, Paus R, Mihm MC. Inhibition of melanogenesis as an adjuvant strategy in the treatment of melanotic melanomas: Selective review and hypothesis. Anticancer Res 1998;18:3709-15.
[Google Scholar]
|
Fulltext Views
2,827
PDF downloads
2,272





