Translate this page into:
Pregnancy and varicella infection: A resident's quest
2 Department of Medicine, PGIMS, Rohtak, India
Correspondence Address:
Sangita Ghosh
42\136, New Ballygunge Road, Kolkata - 39
India
| How to cite this article: Ghosh S, Chaudhuri S. Pregnancy and varicella infection: A resident's quest. Indian J Dermatol Venereol Leprol 2013;79:264-267 |
Introduction
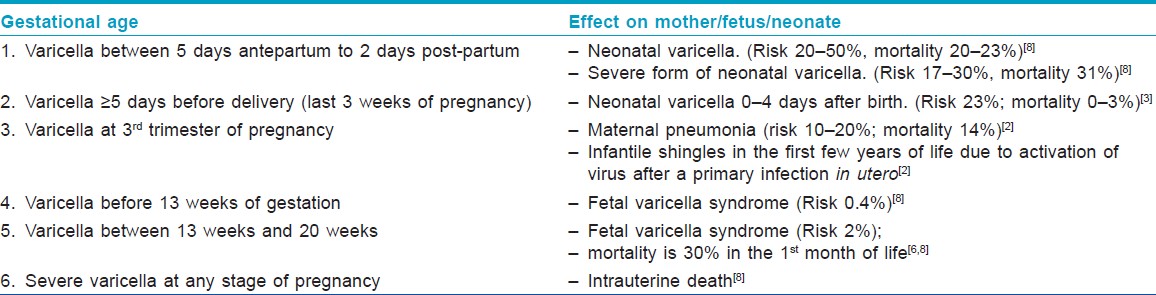
Varicella, or chicken pox, confers lasting immunity and second attacks are uncommon, especially in immunocompetent individuals. Women who reach their child-bearing age without developing immunity to varicella have a small but finite risk of developing chicken pox during pregnancy (0.05-0.07%). [1] Incidence of varicella in pregnancy is calculated to be 2-3/1000 pregnancies. [1] For the mother, the risk of severe illness is greatest after mid pregnancy when she is relatively immunocompromised. For the fetus, the risk of congenital infection is greatest when maternal infection occurs in the first or second trimester.
Maternal Complications Due to Varicella Infection
Although varicella infection is much less common in adults than in children, in persons >15 years of age and children under 1 year of age, it tends to be more severe with a higher rate of complications like pneumonia, hepatitis and encephalitis, and is associated with higher mortality. Pregnant women who contract varicella in the last trimester are at a higher risk of severe pneumonia and death. Pneumonia can occur in up to 10-20% of pregnant women with chicken pox, and the severity of these complications seems to increase in later gestation leading to a mortality rate of 14%, whereas in the general population, varicella pneumonia has a mortality rate of 10-11%. [2],[3],[4] Risk factors for the development of varicella pneumonitis in pregnancy include third trimester infection, cigarette smoking, chronic obstructive lung disease, history of taking systemic steroid in the preceding 3 months or immunosuppression or more than 100 skin lesions or hemorrhagic lesions. [2],[5]
How to Prevent Maternal Varicella?
Pregnant women who have never had varicella infection in the past and/or have not received varicella vaccine, or in whom varicella antibody serology (immunoglobulin gamma) is negative, are considered non-immune. A previous history of chicken pox infection is 97-99% predictive of the presence of serum varicella antibodies. [2]
Varicella zoster immunoglobulin (VZIG), which is a disease-specific immunoglobulin prepared by pooling plasma of donors with high levels of varicella zoster antibody, is recommended as post-exposure prophylaxis for non-immune pregnant women. It has been shown to lower varicella infection rates if administered within 72-96 h after exposure. [1] Protection is estimated to extend through 3 weeks, which corresponds with the half-life of the immunoglobulin. VZIG has no therapeutic benefit once chicken pox has already developed. Aciclovir, as preventive therapy, has been suggested by some authors and is best given on the seventh day post-exposure, but the prophylactic role of this drug in chicken pox is yet to be established. [3]
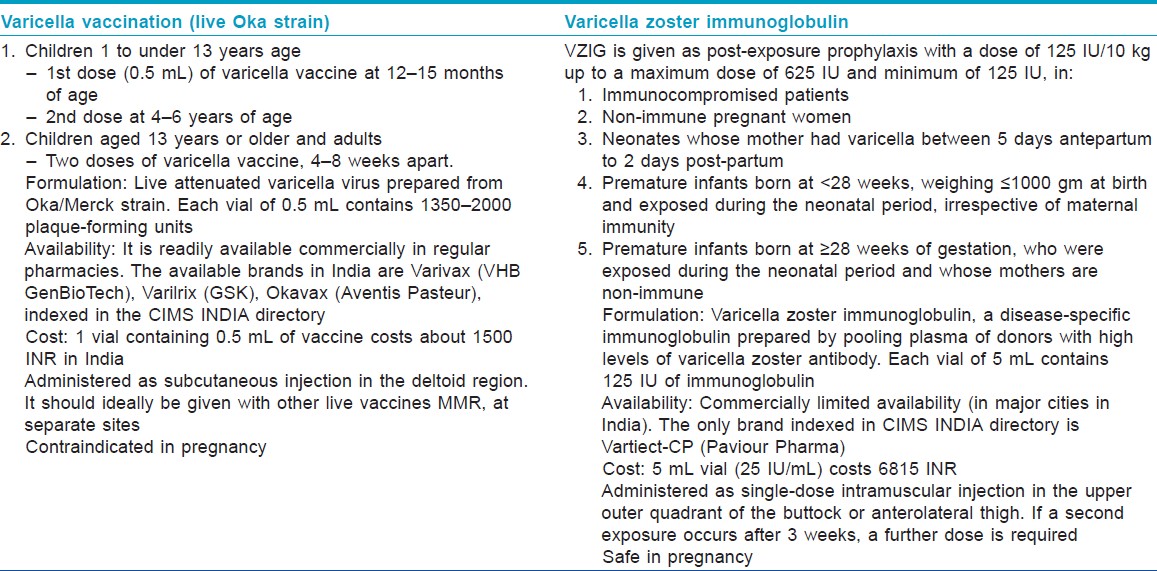
Varicella vaccine (Varivax) contains live attenuated virus derived from the Oka strain. It is not recommended for pregnant women or for those expected to be pregnant in the next 1 month. However, termination of pregnancy should not be recommended in case of inadvertent vaccination during pregnancy. [6] Varicella vaccination pre-pregnancy (at least 1 month prior conception) or post-partum can be considered for women who are found to be seronegative for VZV IgG before pregnancy or in the post-partum period [Table - 1].
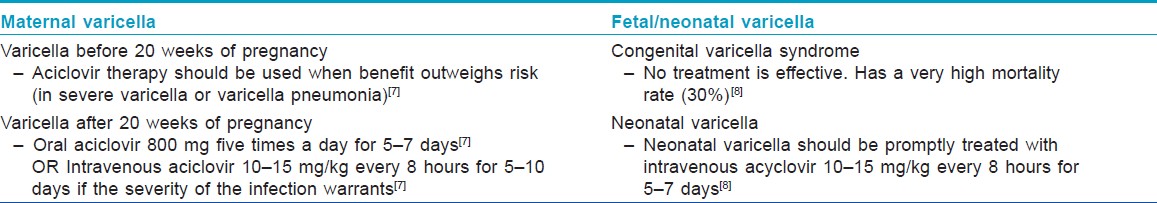
How to Manage Maternal Varicella Infection?
Women with significant varicella infection should be treated with oral aciclovir 800 mg five times a day for 7 days starting within 24-72 hours of onset of rash. [3] Aciclovir is a pregnancy category B drug, and, before 20 weeks of gestation, should be used when potential benefits outweigh risk. [7] In case of progression to varicella pneumonitis, if the severity of infection warrants, intravenous aciclovir (10-15 mg/kg, every 8 hours for 5-10 days) can be considered. [4] Fetal ultrasound scan 5 weeks after the primary infection and appropriate follow-up is recommended to all pregnant women who develop varicella during the first or second trimester to screen for fetal abnormalities. They should be advised to avoid contact with other pregnant women and neonates during the period of communicability, which starts 1-2 days prior to the eruption, until the lesions have all crusted, which usually starts 5 days after the onset of rash. Symptomatic treatment and hygiene is advised to prevent secondary bacterial infection of the lesions.
Fetal Complications of Maternal Varicella
Fetal effects of varicella can manifest as either "Fetal or congenital varicella syndrome" (embryopathy) or neonatal varicella (no embryopathy, but chicken pox infection within the first 10-12 days of life). The implications of primary maternal varicella on her baby vary with the gestational age at infection.
Maternal Varicella in the First and Second Trimesters
"Fetal varicella syndrome" develops in <2% of the babies born to mothers infected with varicella between 7 and 28 weeks of pregnancy, through transplacental infection, and it has a mortality rate of 30% in the first month of life. [6] It does not occur at the time of initial fetal infection but results from a subsequent herpes zoster reactivation in utero and only occurs in a minority of the infected fetuses. [3] Clinically, the newborn presents with low birth weight (LBW), cutaneous scars in a dermatomal distribution, papular lesions resembling connective tissue nevi, ocular abnormalities like cataract, microphthalmia and chorioretinitis, bone and muscle hypoplasia, neurological abnormalities like mental retardation, seizures, hydrocephalus, cortical atrophy and microcephaly, Horner′s syndrome, nystagmus and dysfunction of bladder and bowel sphincters. [6]
Maternal Varicella in the Third Trimester
When maternal infection occurs in the last 3 weeks to >5 days before delivery, there is a significant risk (23%) of neonatal varicella despite high titers of passively acquired maternal antibody. [3] The route of infection can be transplacental, ascending vaginal or can result from direct contact with lesions during or after delivery. Transplacentally transmitted infection manifests in the first 10-12 days of life, whereas chicken pox after that time is most likely acquired by post-natal infection. [5]
If the onset of varicella in the mother is 5 days before delivery to 2 days after delivery, an estimated 20-50% of the newborns contract "neonatal varicella," and 30% of them develop "severe or fulminant neonatal varicella" with disseminated cutaneous lesions and visceral involvement [Figure - 1]. This period correlates with the development of maternal IgG; hence, the neonate does not get enough time to acquire passively transferred maternal antibody and there is also a relative immaturity of the neonatal immune system. [8] In that case, the neonate should be given prophylactic VZIG immediately after birth. With the use of VZIG, the mortality rate has declined from 31% to 7% among neonates with severe varicella infection. [9],[10] Aciclovir therapy should be administered promptly within 24 hours of the onset of rash as it reduces the duration and severity of chicken pox at a dosage of 10-15 mg/kg every 8 h intravenously for 5-7 days. [8],[11] Date of delivery may be post-poned allowing maternal antibodies to pass the placental barrier. No controlled study has yet established the effectiveness of aciclovir or valacyclovir for post-exposure prophylaxis to neonates or pregnant women [3] [Table - 2] and [Table - 3].
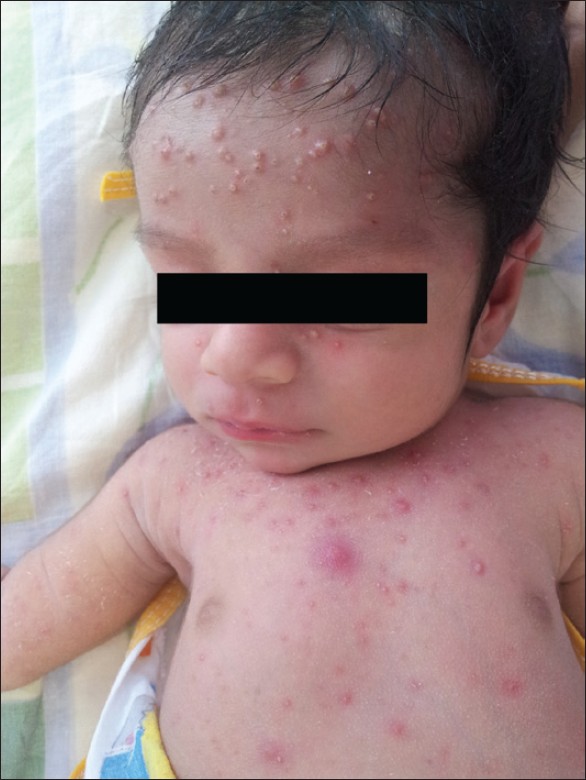 |
| Figure 1: "Neonatal varicella" in a 6-day-old child whose mother had chicken pox 3 days before delivery |
Conclusion
Chicken pox during pregnancy can have gestational age-specific adverse outcomes both in the mother and in the fetus. Varicella vaccine is contraindicated in pregnancy. Non-immune exposed expectant mothers can be offered VZIG as prophylaxis before infection occurs. Once infection occurs, oral or intravenous aciclovir can be advised depending on the severity of infection. Neonatal varicella infection should be treated promptly with intravenous aciclovir.
| 1. |
Enders G, Miller E, Cradock-Watson J, Bolley I, Ridehalgh M. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet 1994;343:1548-51.
[Google Scholar]
|
| 2. |
Harger JH, Ernest JM, Thurnau GR, Moawad A, Momirova V, Landon MB, et al. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis 2002;185:422-7.
[Google Scholar]
|
| 3. |
Tan MP, Koren G. Chickenpox and pregnancy: Revisited. Reprod Toxicol 2005;21:410-20.
[Google Scholar]
|
| 4. |
Shrim A, Koren G, Yudin MH, Farine D; Maternal Fetal Medicine Committee. Management of varicella infection (chickenpox) in pregnancy. J Obstet Gynaecol Can 2012;34:287-92.
[Google Scholar]
|
| 5. |
Nathwani D, Maclean A, Conway S, Carrington D. Varicella Infections in pregnancy and the newborn. J Infect 1998;36:59-71.
[Google Scholar]
|
| 6. |
Pastuszak AL, Levy M, Schick B, et al. Outcome after maternal varicella infection in the first 20 weeks of pregnancy. N Engl J Med 1994;330:901-5.
[Google Scholar]
|
| 7. |
Ratanajamit C, Vinther Skriver M, Jepsen P, Chongsuvivatwong V, Olsen J, Sørensen HT. Adverse pregnancy outcome in women exposed to acyclovir during pregnancy: A population-based observational study. Scand J Infect Dis 2003;35:255-9.
[Google Scholar]
|
| 8. |
Sauerbrei A, Wutzler P. Neonatal varicella. J Perinatol 2001;21:545-9.
[Google Scholar]
|
| 9. |
Meyers JD. Congenital varicella in term infants: risk reconsidered. J Infect Dis 1974;129:215-7.
[Google Scholar]
|
| 10. |
Miller E, Cradock-Watson JE, Ridehalgh MK. Outcome in newborn babies given anti-varicella-zoster immunoglobulin after perinatal maternal infection with varicellazoster virus. Lancet 1989;2:371-3.
[Google Scholar]
|
| 11. |
Dunkle LM, Arvin AM, Whitley RJ, Rotbart HA, Feder HM Jr, Feldman S, et al. A controlled trial of acyclovir for chickenpox in normal children. N Engl J Med 1991;325:1539-44.
[Google Scholar]
|
Fulltext Views
16,037
PDF downloads
4,911





