Translate this page into:
Alopecia areata in a patient receiving infliximab
2 Department of Gastroenterology, Hospital Donostia, San Sebastian, Spain
Correspondence Address:
Nerea Ormaechea-P�rez
Paseo Doctor Begiristain, Department of Dermatology, Hospital Donostia, San Sebastian, CP: 20014
Spain
| How to cite this article: Ormaechea-P�rez N, L�pez-Pesta�a A, Mu�agorri-Santos AI, Moreno AJ, Tuneu-Valls A. Alopecia areata in a patient receiving infliximab. Indian J Dermatol Venereol Leprol 2013;79:529-531 |
Sir,
In the last few years, several tumor necrosis factor alfa (TNF-α) antagonists are being used for the treatment of chronic immune inflammatory disorders. With their increasing use and longer follow-up periods, adverse effects including a wide-spectrum of autoimmune diseases are emerging as adverse events. We report a case of alopecia areata (AA) in a patient receiving infliximab for Crohn′s disease.
A 36-year-old white woman with Crohn′s disease of 2 years duration was treated with infliximab in monotherapy as first-line therapy at an intravenous dose of 5 mg/kg, according to the standard schedule, for the last 9 months. After the fourth infusion, she developed a non-scarring hair loss involving sides and occiput (ophiasis area), eyebrows, and eyelashes [Figure - 1]. She had no nail changes, clinical features of collagen vascular disorders, nor personal or family history of AA. Blood analysis, including thyroid hormones, was normal except for positive anti-nuclear antibodies of 1/1280. A biopsy specimen of the scalp showed peribulbar lymphocytic infiltrates with a reduced number of hairs consistent with AA [Figure - 2]. Therapy with infliximab was discontinued, and she was treated with methotrexate at a weekly dose of 25 mg, in order to treat her Crohn′s disease and the AA, getting total recovery of the scalp hair in 2 months [Figure - 3]. Furthermore, the anti-nuclear antibodies became negative. She continued with methotrexate for 4 months for her inflammatory bowel disease, with no further alopecia or other adverse event during 12 months of follow-up.
 |
| Figure 1: Non-scarring alopecic patches involving ophiasis area and eyebrows |
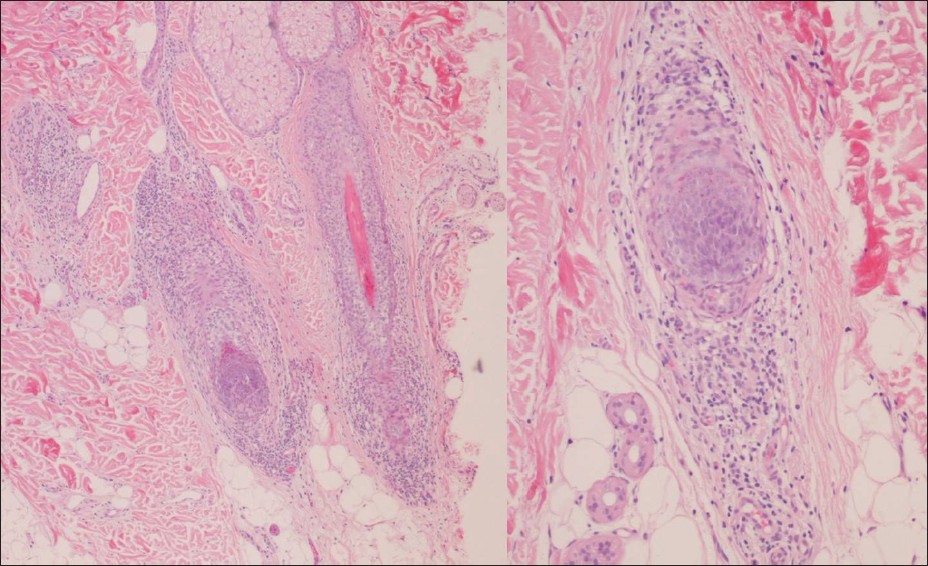 |
| Figure 2: Scalp biopsy histology showing peribulbar lymphocytic infiltrates (H and E, × 40) |
 |
| Figure 3: Almost complete re-growth after infliximab cessation |
AA is an autoimmune inflammatory disease, in which T lymphocytes play a central pathogenetic role. It is postulated that CD4+ and CD8+ T cells directed against hair bulb autoantigens lead to non-scarring hair loss. Nevertheless, some authors have suggested a complex and contradictory role of TNF-α in its pathogenesis. In vitro studies have shown that TNF-α and interleukin-1-β are potent inhibitors of hair follicle growth, by causing distorsion of the dermal papilla, vacuolization, and abnormal keratinization of the matrix. However, anti-TNF-α agents have shown no efficacy in the treatment of AA. [1]
Infliximab is a chimeric monoclonal antibody against TNF- α that is used to treat autoimmune diseases. However, it has been associated with the development of other autoimmune disorders such as psoriasis, granuloma annulare, or vitiligo. It is proposed that TNF-α blocking switches off the primary disease′s inflammatory pathway, moving the unblocked proximal inflammatory response into an alternative signaling pathway. Depending on individual genetic susceptibility, this pathway could clinically manifest as another immune-mediated disease, such as AA. [2]
Several AA cases have been described during anti-TNF-α therapy. In the literature reviewed, we found 5 cases of AA after therapy with infliximab: [3],[4],[5],[6],[7] Two cases in patients with rheumatoid arthritis, one in a patient with psoriasis pustulosa, and other two in patients with ankylosing spondylitis. Anti-nuclear antibodies titer was available in one case, [4] and was negative.
AA is usually associated with other autoimmune disorders such as vitiligo and thyroid disease, but there is little data in the literature about the association of AA and Crohn′s disease. [8] Considering that the patient had no history of AA, that AA happened after beginning treatment with infliximab and that it resolved when the anti-TNF-α therapy was discontinued, we believe that in our patient, infliximab might have had a predominant role in triggering the AA. In addition, the fact that the autoantibodies raised during treatment with infliximab, and the decrease of them when the therapy was discontinued strengthens our hypothesis that infliximab may have caused this autoimmune phenomenon. Taking into account that different TNF- α inhibitors have shown to induce diverse biological and clinical effects, AA may have been induced by infliximab, but other TNF- α inhibitors could not have led to the disease. On the other hand, MTX may have had some influence in the clinical course of AA. In conclusion, we describe the first case of AA during infliximab therapy in a patient with Crohn′s disease.
| 1. |
Beccastrini E, Squatrito D, Emmi G, Fabbri P, Emmi L. Alopecia areata universalis during off-label treatment with infliximab in a patient with Behçet disease. Dermatol Online J 2010;16:15.
[Google Scholar]
|
| 2. |
Ferran M, Calvet J, Almirall M, Pujol RM, Maymo J. Alopecia areata as another immune-mediated disease developed in patients treated with tumour necrosis factor- α blocker agents. J Eur Acad Dermatol Venereol 2011;25:479-84
[Google Scholar]
|
| 3. |
Ettefagh L, Nedorost S, Mirmirani P. Alopecia areata in a patient using infliximab: New insights into the role of tumor necrosis factor on human air follicles. Arch Dermatol 2004;140:1012
[Google Scholar]
|
| 4. |
Tosti A, Pazzaglia M, Starace M, Bellavista S, Vincenzi C, Tonelli G. Alopecia areata during treatment with biologic agents. Arch Dermatol 2006;142:1653-4.
[Google Scholar]
|
| 5. |
Fabre C, Dereure O. Worsening of alopecia areata and de novo occurrence of multiple halo nevi in a patient receiving infliximab. Dermatology 2008;216:185-6.
[Google Scholar]
|
| 6. |
Hernández MV, Nogués S, Ruiz-Esquide V, Alsina M, Cañete JD, Sanmartí R. Development of alopecia areata alter biological therapy with TNF-alpha blockers: Description of a case and review of the literature. Clin Exp Rheumatol 2009;27:892-3.
[Google Scholar]
|
| 7. |
Nakagomi D, Harada K, Yagasaki A, Kawamura T, Shibagaki N, Shimada S. Psoriasiform eruption associated with alopecia areata during infliximab therapy. Clin Exp Dermatol 2009;34: 923-4.
[Google Scholar]
|
| 8. |
Ganzetti G, Campanati A, Offidani A. Alopecia areata: A possible extraintestinal manifestation of Crohn's disease. J Crohns Colitis 2012;6:962-3.
[Google Scholar]
|
Fulltext Views
4,260
PDF downloads
2,059





