Translate this page into:
Co-reactivation of varicella zoster virus and herpes simplex virus with disseminated cutaneous lesions in a lymphoma patient
Correspondence Address:
A S Krishnaram
Professor and Head of Department, Department of Dermatology, Madurai Medical College, Madurai, Tamil Nadu - 625 001
India
| How to cite this article: Krishnaram A S, Sachan Y, Rani G G. Co-reactivation of varicella zoster virus and herpes simplex virus with disseminated cutaneous lesions in a lymphoma patient. Indian J Dermatol Venereol Leprol 2013;79:709-711 |
Sir,
65-year-old male, known case of non-hodgkin lymphoma completed four cycles of chemotherapy (CHOP) two months back, presented with generalized vesicular eruption and constitutional symptoms of five days. Patient had chicken pox one year back. History of vesicular eruption over lips was recorded for over two years with three episodes at intervals of six months, each lasting for 4-5 days. On examination, discrete and grouped vesicles were seen on the face, trunk and upper extremities with relative sparing of lower extremities and acral regions. Few vesicles showed umblication and necrosis. Few pustules were also seen over the back and chest. Discrete vesicles over erythematous base as well as grouped vesicles were also present. Large bullous lesions and grouped vesicles in a zosteriform pattern were seen on left upper back over multiple dermatomes (C7, C8, and T1) [Figure - 1]a. There was puffiness of face and swelling of lips [Figure - 1]b with oral erosion. Based on clinical findings, possibility of disseminated zoster or disseminated herpes simplex infection or co-infection of both at different anatomical sites was considered. Kaposi varicelliform eruption was excluded by absence of a pre-existing dermatoses, lack of dense and close aggregation of vesicles, polymorphous lesion, umblication present only in few vesicles and larger area of body surface involvement.
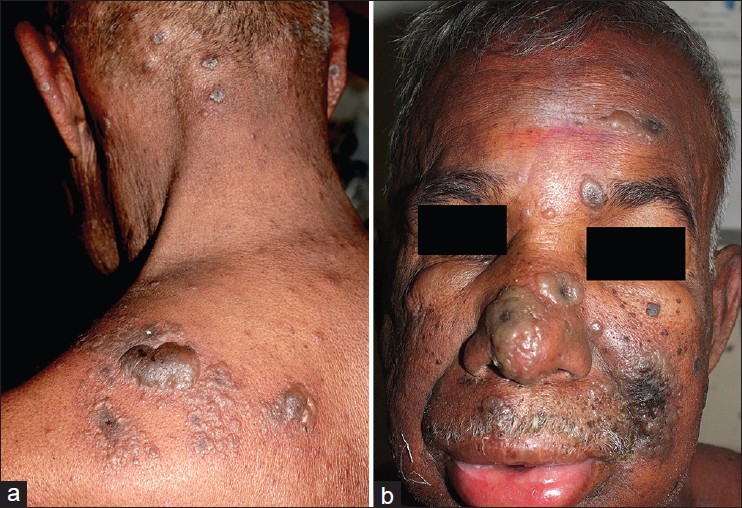 |
| Figure 1: (a) Bullous lesions in a zosteriform pattern. Aberrant vesicles and pustules in the neck. (b) Face showing oedema of the face and lips |
Tzanck smear revealed multiple acantholyic cells and multinucleate giant cells. Uniplex and nested polymerase chain reaction (PCR) for both VZV (Varicella Zoster Virus) and HSV-1 (Herpes Simplex Virus 1) from the vesicular fluid obtained from discrete as well as grouped vesicles over trunk was positive and thus confirmatory of acute co-infection. IgG (Immunoglobulin G) antibody titres for VZV and HSV-1 were 1.2 and 1.3 respectively (titre > 1.0 positive). IgG antibody titres done on parallel specimen of acute sera (obtained on 5 th day of vesicular eruption) and convalescent sera (obtained on 18 th day) showing four-fold rise denoted recent and past infection. IgM (Immunoglobulin M) antibody titre was not done as PCR confirmed the acute co-infection. Also, the lower sensitivity and specificity of IgM antibody titre, its inability to distinguish between HSV-1 and HSV-2 (Herpes Simplex Virus 2), false positivity to other viruses such as EBV and VZV, lower rise in antibody titre in reactivation and inability to distinguish primary from reactivation in the presence of IgG antibodies were other reasons for not considering IgM antibody titres. Complete blood counts, renal and liver parameters, chest radiograph, ultrasound abdomen and pelvis were found normal. Acyclovir 10 mg/kg body weight 8 hourly for 7 days was given parenterally. Skin lesions and constitutional symptoms improved within a week [Figure - 2]a-c. Interestingly, a 23 year old intern at the medical ward developed varicella 10 days post-exposure.
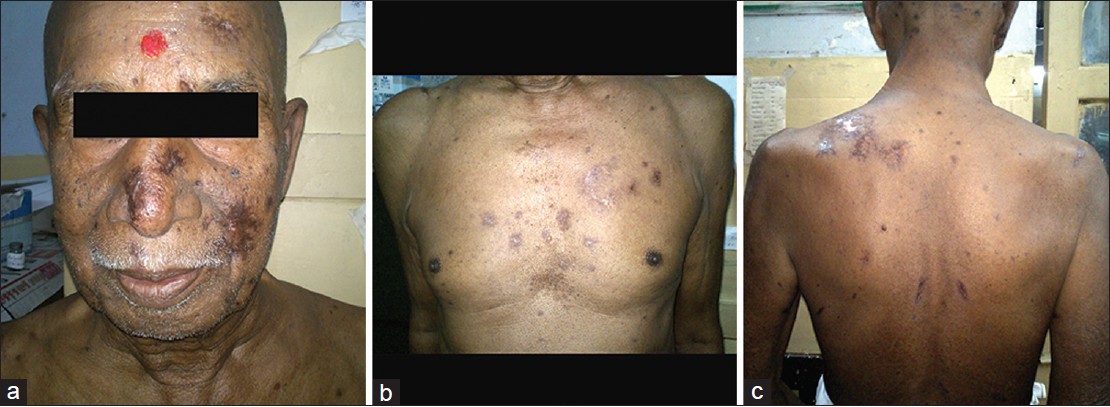 |
| Figure 2: Post therapy picture showing complete healing of lesions- (a) face (b) chest (c) back |
VZV causes both varicella and herpes zoster. Herpes zoster is 20 to 100 times more common in an immunosuppressed individual. [1] In malignancy, especially lymphomas, zoster is complicated by disseminated cutaneous disease and visceral involvement. In 2% to 10% of immunocompromised patients with zoster, disseminated cutaneous disease occurs. [2] Disseminated herpes zoster occurs due to hematogenous dissemination of virus due to delayed immune response. [3] Herpes simplex caused by herpes simplex virus (HSV-1 and HSV-2), presents as discrete groups of painful vesicles on orolabial and genital skin respectively. In an immunosuppressed host severe, extensive, widespread eruption of vesicles, pustules and erosions can occur. Disseminated herpes simplex virus infections may resemble varicella; however, there is often an obvious concentration of lesions at and surrounding the site of the primary or recurrent infection and there may be associated pneumonia, hepatitis and encephalitis. [3]
This case was considered interesting because of the immunocompromised state and disseminated complex vesicular lesions that could represent either of the viral infections, varicella, zoster or herpes simplex. Clinically, the widespread infection lesions at varying stages in few areas on the back, sparing of distal areas seemed suggestive of varicella in an immunosuppressed patient, in whom reinfection is possible. Grouped lesions on the face and upper back, unlikely to be seen in varicella aroused suspicion of disseminated zoster or herpes simplex. Since the zosteriform lesion over the back satisfied the criteria of disseminated herpes zoster which is defined as more than 20 vesicles outside the area of primary or adjacent dermatomes producing a varicella-like eruption, [2] clinically the diagnosis of disseminated zoster was made. Disseminated herpes simplex was also considered as an alternative diagnosis based on the grouped lesions preferentially involving perioral and nasal area, not in a zosteriform pattern. Facial oedema and oral erosion were more suggestive of HSV-1 infection. Usually disseminated herpes simplex in an immunosuppressed host presents with systemic involvement, our patient was stable with no systemic complications.
The lesions of varicella, herpes zoster and herpes simplex are indistinguishable by Tzanck smear or histopathology. Detection of viral DNA in clinical specimens after amplifications by PCR provides the greatest assay sensitivity, very high specificity, and rapid turn-around time. [2] PCR from grouped as well as discrete lesions from the face and back was positive for both VZV and HSV-1. A four-fold rise in IgG antibody titres in the convalescent sera was also seen, for both VZV and HSV-1. Past history of chicken pox and labial vesiculation suggested reactivation of dormant viruses. A final diagnosis of co-reactivation of disseminated zoster and disseminated herpes simplex infection was made. Usually, disseminated herpes simplex has multiple visceral involvement and high mortality. In our case, coinfection with VZV and HSV-1 presented with lesser morbidity and severity and responded readily to treatment with no recurrence even after 6 months of follow up. Development of varicella in the intern 10 days post-exposure indicates high infectivity of disseminated zoster as compared to that of zoster. Patients with zoster are infectious, primarily through direct contact with active lesions. VZV can also be transmitted through an air-borne route, particularly in patients with disseminated zoster increasing the secondary attack rate manifold.
As far as known, around 30 cases of similar co-infection occurring in immunosuppressed as well as immunocompetent patients have been reported worldwide. [4],[5],[6],[7],[8],[9] Interestingly we could find no similar documentation from India. In a study conducted by Giehl KA et al., of 1718 patients reviewed 1.2% was found infected with both HSV and ZVZ at the same body site, with a clinical diagnosis of zoster in 60%, herpes simplex in 20%, and varicella in 10% and erythema muliforme in 5% of cases. [9] Co-reactivation can be attributed to asymptomatic, latent expression of HSV-1 in a patient reinfected with VZV due to the immunocompromised state. Another probable explanation is that reactivation of a neurotropic herpes virus can reactivate another neurotropic virus if both types are present in the same ganglion. [4] Treatment for VZV itself will be sufficient for treating co-infection of HSV/VZV.
This case is being presented for its rarity; unusual co-reactivation of VZV and HSV-1, disseminated cutaneous lesions and infectivity of disseminated zoster paralleling that of varicella and finally for its documentation from India.
| 1. |
Stephen ES, Michael NO, Kenneth ES, Varicella, Herpes Zoster. Dermatology in general medicine. In: Wolff K, Golsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. 7 th ed. New York: McGraw Hill; 2008. p. 1885-98.
[Google Scholar]
|
| 2. |
Madkan V, Sra K, BranHey J, Carrasco D, Mendoza N, Tyring SK. Human herpes viruses. Dermatology. In: Bolognia JL, Lorizzo JL, Rpini RP, editors. 2 nd ed. New Delhi: Elsevier; 2009. p. 1199-1208.
[Google Scholar]
|
| 3. |
Talwar S. Herpes zoster associated with varicelliform eruptions. Indian J Dermatol Venerol Leprol 1991;57:2.
[Google Scholar]
|
| 4. |
Danielson PL, Schonning K, Larsen HK. Herpes simplex virus type 1 in a patient with herpes zoster. Ugeskr Laeger 2012;174:425-6.
[Google Scholar]
|
| 5. |
Curley MJ, Hussein SA, Hassoun PM. Disseminated herpes Simplex virus and varicella zoster virus coinfection in a patient taking thalidomide for relapsed multiple myeloma. J Clin Microbiol 2002;40:2302-4.
[Google Scholar]
|
| 6. |
Nikkels AF, Frère P, Rakic L, Fassotte MF, Evrard B, De Mol P, et al. Simultaneous reactivation of herpes simplex virus and varicella-zoster virus in a patient with idiopathic thrombocytopenic purpura. Dermatology 1999;199:361-4.
[Google Scholar]
|
| 7. |
Gibney MD, Leonardi CL, Glaser DA. Concurrent herpes simplex and varicella-zoster infection in an immunocompromised patient. J Am Acad Dermatol 1995;33:126-9.
[Google Scholar]
|
| 8. |
Park HH, Lee MH. Concurrent reactivation of varicella zoster virus and herpes simplex virus in an immunocompetent child. J Korean Med Sci 2004;19:598-600.
[Google Scholar]
|
| 9. |
Giehl KA, Muller-Sander E, Rottenkolber M, Degitz K, Volkenandt M, Berking C. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. J Eur Acad Dermatol Venereol 2008;22:722-8.
[Google Scholar]
|
Fulltext Views
5,598
PDF downloads
1,864





