Translate this page into:
Patch testing in cutaneous adverse drug reactions: Methodology, interpretation, and clinical relevance
2 Department of Dermatology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Correspondence Address:
Vikram K Mahajan
Department of Dermatology, Venereology and Leprosy, Dr. R. P. Government Medical College, Kangra, (Tanda) - 176 001, Himachal Pradesh
India
| How to cite this article: Mahajan VK, Handa S. Patch testing in cutaneous adverse drug reactions: Methodology, interpretation, and clinical relevance. Indian J Dermatol Venereol Leprol 2013;79:836-841 |
Introduction
Adverse drug reaction is a noxious and unintended response to a drug at normal doses used in humans for prophylaxis, diagnosis or therapy of a disease or for the modification of physiological function (WHO). [1] Cutaneous adverse drug reactions are a frequent problem in clinical practice and comprise 1-2% of outdoor and 5-10% of indoor patients in dermatology practice. [2] It is often difficult to pinpoint the offending drug from temporal correlation/history alone as most affected patients are on multiple medications, and the clinical picture is often not diagnostic. Although, re-challenge/provocation tests, intradermal tests or skin prick tests are of significant value in identifying the culprit drug, they are time consuming, need expertise or may even re-precipitate life-threatening adverse drug reaction that may raise ethical issues. Availability of basophil degranulation/lymphocyte activation tests remains limited, has low sensitivity/specificity and may even be negative during acute stage of drug hypersensitivity. Radioallergosorbent test for drug specific IgE, histamine release test, and passive hemagglutination test with sensitivity/specificity nearly similar to skin tests have limited availability/applicability in routine clinical practice. An increased focus has been witnessed in recent years on the utility of drug patch test in cutaneous adverse drug reactions since it is easy to perform, relatively safe, and the only in-vivo challenge test available. Hence, it becomes imperative to revisit methodology, interpretation, and clinical relevance of drug patch testing.
Mechanisms of Adverse Drug Reactions
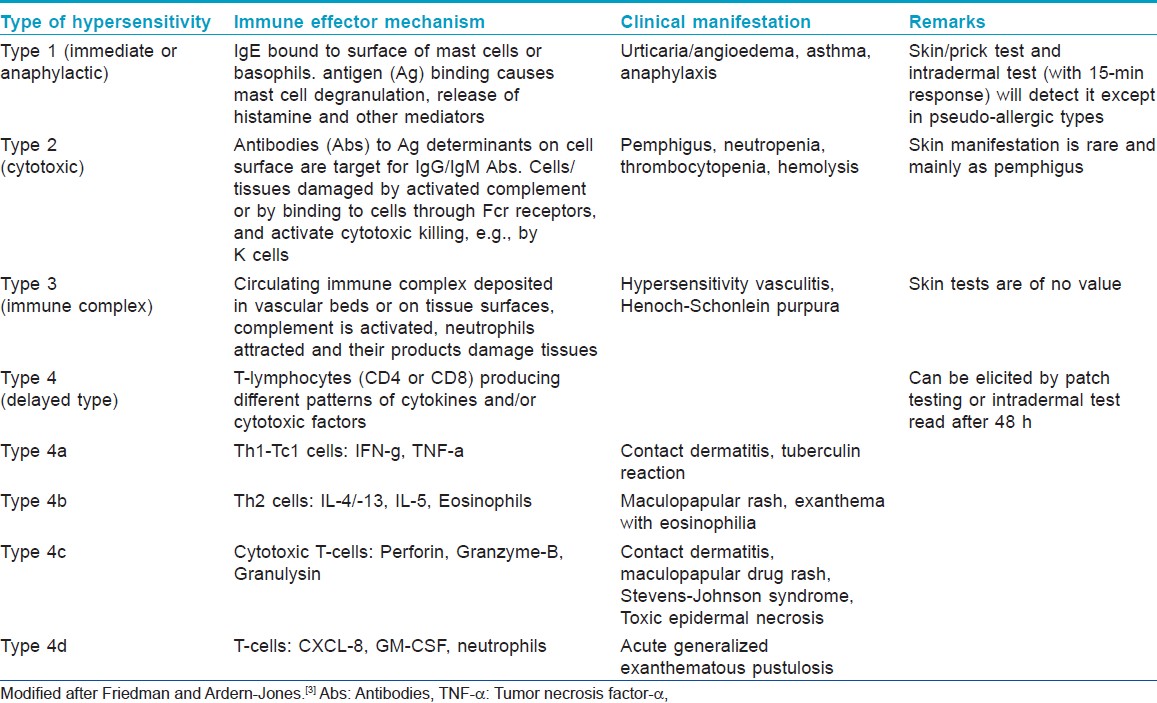
It will be prudent here to recapitulate briefly the mechanisms involved in adverse drug reactions. The majority (nearly 95%) of adverse drug reactions are Type A (augmented) reactions which are dose-dependent and predictable from the primary and secondary drug pharmacology while Type B (Bizarre) reactions are idiosyncratic, unpredictable from drug pharmacology, and determined by patient-specific susceptibility factors. [3] They can be "non-immune mediated (drug intolerance)" due to inadequate or imperfect metabolic detoxification leading to hemolysis, bone marrow toxicity or neurotoxicity from toxic metabolites or "pseudo-allergic" due to histamine, leukotrienes, or other mediators released from direct basophil/mast cell de-granulation. These are often difficult to distinguish from true immunologically mediated immediate type allergic responses (e.g., asthma, anaphylaxis, and urticaria/angioedema-like reactions due to drugs like opiates, muscle relaxants or radio contrast media). [Table - 1] lists four main classes of immune effecter mechanisms involved in immune mediated Type B (immunologic) reactions which are relevant for drug patch testing. [3]
Basic Principles, Methodology, and Reporting of Results
The basic principles and methodology for drug patch test remains same as that in patch testing for contact dermatitis. The T-cells get activated by adaptive immune mechanism from hapten-antigen presenting cells (APCs) and human leukocyte antigen (HLA) complex (HLA-class I and/or II). For this, drug molecule or the metabolite becomes a hapten that binds with protein to form an antigen which then is processed by APCs. Once T-helper cells get activated, they proliferate to produce clones of memory/effector T-cells with specificity for that immunogen with ability to activate immune effector mechanism (immunological memory) or they in turn help B cells to produce antibodies (IgA, IgG, IgE). It requires 7-10 days to develop immunological memory (sensitization phase) meaning thereby that one must have continuous exposure for this much period to the offending drug for tissue damaging hypersensitivity to develop (elicitation phase). Re-exposure to the culprit drug will elicit similar clinical reaction pattern in previously sensitized individuals. This is the background principle for drug patch testing.
Finn chamber method is used for drug patch testing and test units are applied over non-hairy, upper back after cleansing with ethanol without rubbing [Figure - 1]. For drug reactions such as fixed drug eruptions (FDE), Stevens-Johnson syndrome-toxic epidermal necrolysis (SJS-TEN), intertriginous/flexural exanthem less affected skin sites may remain negative while, initially affected skin sites elicit more positive responses and should be selected for patch testing. [4],[5] The patient is instructed to leave the patches in place for next 48 h (2 days), avoid rubbing, scratching, or wetting them. Patches are removed and marked clearly and responses are read 1-2 h after letting the skin regain its normal contour and the non-specific skin irritation subsides. Patch test results can be negative on day 2 (D2) but, positive when read on day 4 (D4). Readings should be made at 20 min for urticaria or anaphylaxis-like immediate adverse drug reactions from betalactam antibiotics, neomycin, gentamycin, bacitracin, diclofenac. For drug photo-patch test, performed in drug induced photodermatitis, photo allergic/toxic reactions, two sets of patch tests are applied. One set of drug photo-patch test is irradiated with ultraviolet (UV)-A (5 or 10 J/cm 2 ) at 24 or 48 h (D1 or D2). [6] It will be pertinent to mention here that the protocol for reading of patch test results in cases of FDE is not different. [7] For obvious reasons treatment with systemic corticosteroids or immunosuppressive drugs is required to be stopped at least 1 month before patch testing. [6] Similarly, topical corticosteroids should not be used over test sites for at least 2 weeks prior to the patch test; topical corticosteroids applied in large doses even away from the test site may have same effect as low doses of systemic corticosteroids. [8] Strong UV-exposure (e.g., exposure at seaside) prior to drug patch testing will diminish test reactivity. [6] However, anti-histamines do not interfere with test results and can be allowed during/prior to drug patch testing (cf skin prick test).
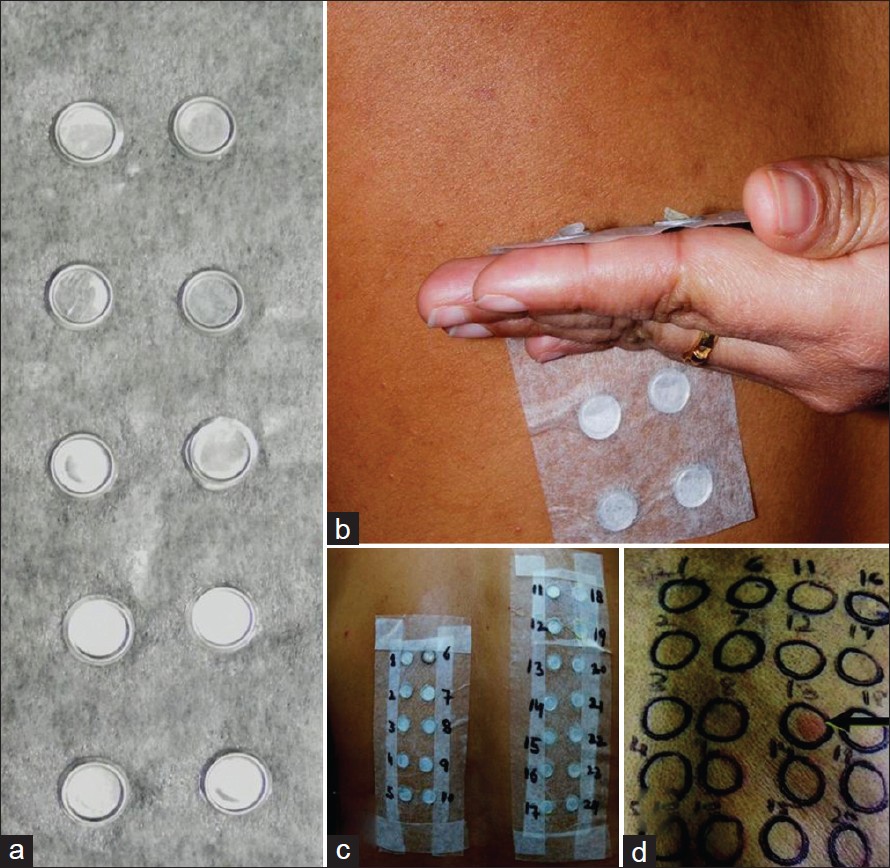 |
| Figure 1: Finn chambers, 8 mm diameter and 0.5 mm deep, made of stiff aluminum and placed on a strip of adhesive (micropore®) tape (a); and placed over upper back with a uniform pressure applied in below upward direction for tight apposition to the skin (b); multiple test preparations placed in Finn chambers and the way they appear after application (c); A strong positive (2+) reaction (d) |
The results are then read at D2, at D3 and another reading is made at D7 if initial results are negative. Results are reported according to International Contact Dermatitis Research Group criteria [9] as: No reaction (0), Doubtful reaction; faint erythema (?), weak positive; palpable erythema, infiltration, papules (1+), strong positive [Figure - 1]d; erythema, infiltration, papules, vesicles (2+), extreme positive reaction; intense erythema and infiltration and coalescing vesicles (3+), irritant reaction (IR) of different types, and not tested (NT).
Specific Issues about Drug Patch Testing
The selection of a patient for drug patch testing is important as the final results depend upon a number of factors (vide infra) which can influence interpretation and usefulness of this procedure.
Clinical types of cutaneous adverse reactions
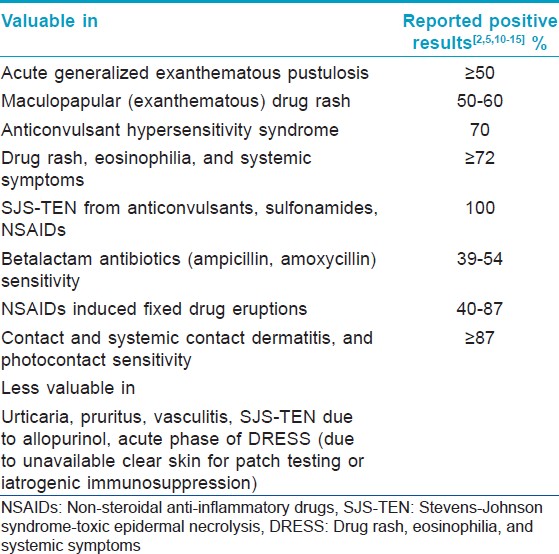
As not all cutaneous adverse reactions will elicit positive results, drug patch testing is suitable for investigating only a select type of drug reactions [Table - 2]. [Figure - 2], [Figure - 3], [Figure - 4] depict serious clinical forms of adverse cutaneous drug reactions and potential cases of drug patch testing. A review of published reports suggests that, drug patch testing is particularly useful in cases of acute generalized exanthematous pustulosis (AGEP, ≥50%), maculopapular drug rash (50-60%), anticonvulsant hypersensitivity syndrome (70%), drug rash, eosinophilia, and systemic symptoms (DRESS, ≥72%), and SJS-TEN (up to 100%), betalactam antibiotics (ampicillin, amoxycillin) sensitivity (39-54%), non-steroidal anti-inflammatory drugs (NSAIDs) induced FDE (40-87%), contact and systemic contact dermatitis, and photocontact sensitivity (≥87%). [2],[5],[10],[11],[12],[13],[14],[15] On the other hand, it is less valuable in cases of immediate type of drug reactions, pruritus, vasculitis, some cases of SJS-TEN (e.g., due to allopurinol), and photodermatitis from systemic drugs. [2],[5],[12],[13],[16] Patch testing is more reliable for eczematous type and (systemic) contact dermatitis or anti-convulsant hypersensitivity reactions, which in most instances is due to anti-convulsants, NSAIDs, sulfonamides or betalactum antibiotics. [15]
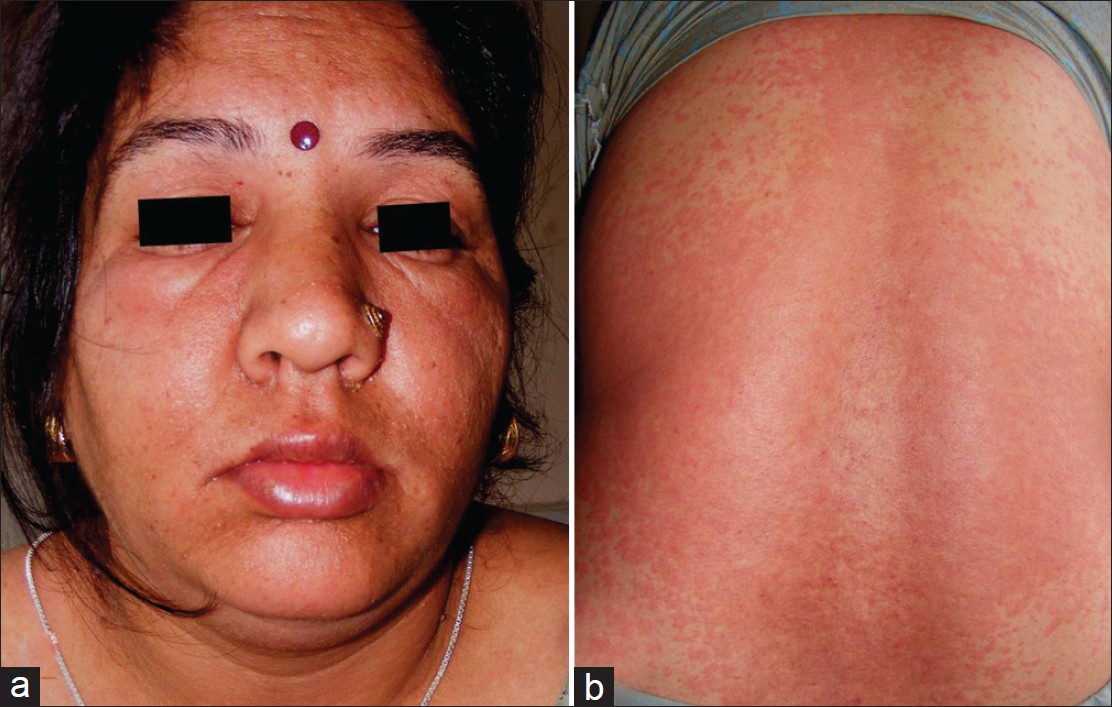 |
| Figure 2: A patient of generalized exanthematous drug rash of anticonvulsant hypersensitivity syndrome (from carbamazapine) characterized by moderate fever, facial and peri-orbital edema, and prominently pruritic maculopapular rash that occurred during first 2 weeks of drug intake (may appear even 10-14 days after stopping it). She also eventuated to AGEP (note: Pustular lesions over face); it can also progress to DRESS if multi-organ involvement, lymphadenopathy and eosinophilia develop |
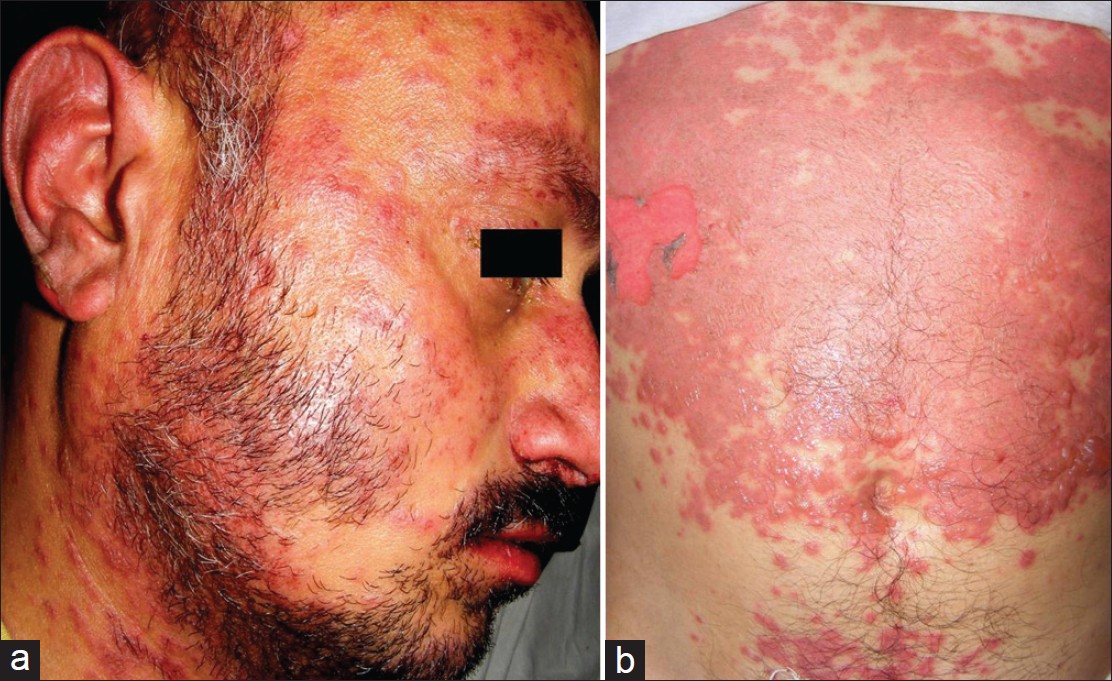 |
| Figure 3: A patient of Stevens-Johnson syndrome (from phenytoin) showing macular erythema, blisters, and detachment of skin (involving 10% body surface area) and mucosae (a); atypical targetoid spots and bullae are also seen over face and beyond large lesion over trunk (b) |
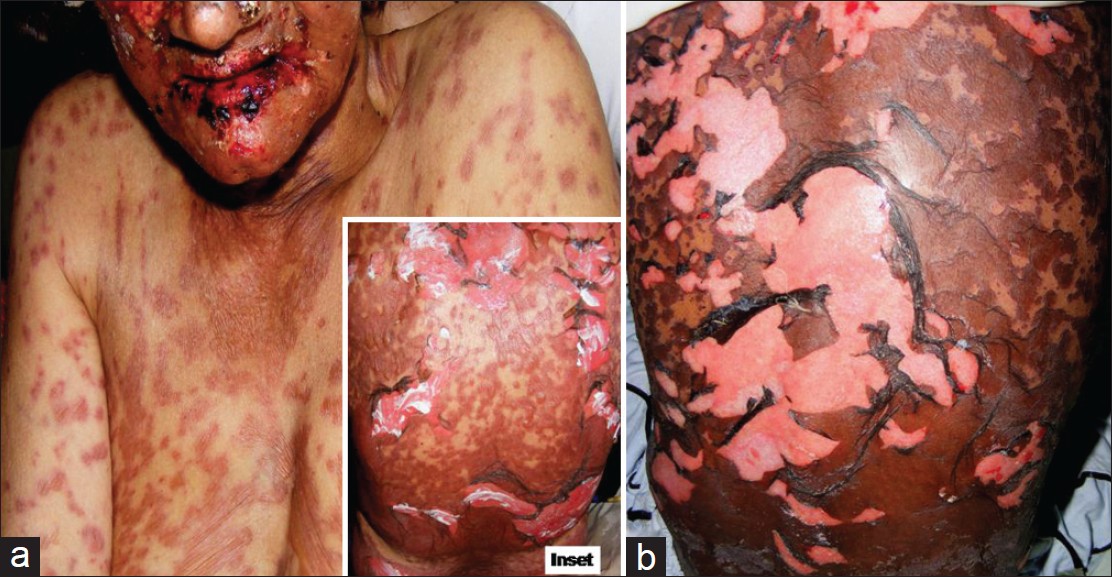 |
| Figure 4: A patient of Stevens-Johnson syndrome-toxic epidermal necrolysis overlap (from diclofenac) showing macular erythema, blisters, and detachment of epidermis from the dermis (between > 10% and 30% body surface area) and mucosae, inset shows lesions over back (a); atypical targetoid spots are also seen over chest. SJS eventuate through SJS-TEN overlap may develop more serious toxic epidermal necrolysis with wide-spread (>30% body surface area) skin/mucosal detachment and multi-organ involvement (b) |
Putative drug(s)
The most immunologic (Type B) drug reactions are due to anti-convulsants (carbamazapine, phenytoin, phenobarbitone, or lamotrigine), sulfones, sulfonamides, and co-trimaxozole, NSAIDs (paracetamol, diclofenac, piroxicam, metamizole), betalactum antibiotics (amoxicillin, ampicillin), and tetrazepam. [3],[4],[5],[10],[13] The list of putative drugs is expanding by the day; allopurinol, minocycline, nevirapine, abacavir are few recent additions. Less common causes of drug reactions are due to acyclovir, valaciclovir, diazepam, clobazam, terbinafine, fluoroquinolones, paracetamol, glucocorticoids, pseudoephedrine, heparin, captopril, and radio contrast media. [5],[7],[17] Although, allopurinol has emerged second most common (first being anticonvulsants) cause of SJS-TEN, patch test is usually negative. [12],[18] [Table - 3] lists some common drugs used for drug patch testing, however, there is no consensus protocol that includes all drugs for testing.

Test preparations, test vehicles, and controls
Pure drug form should be used for patch testing whenever possible in concentrations of 1-10% in petrolatum, water or alcohol. [3],[5],[11],[19] Alternatively, liquid preparations or powder obtained from capsules or pulverized tablets/pills can be used at concentrations up to 30% or as is. [3] Drugs like carbamazapine, pseudoephedrine, acyclovir may re-elicit drug reaction during patch test therefore it is recommended to use 1% dilution and up to 10% when initial responses are negative. [3] Excipients, gel jacket of the capsule (as is), etc., are also required to be included for patch testing. Although petrolatum remains the most preferred vehicle irrespective of the nature of test substance, it is crucial to use water and alcohol as vehicles additionally for preparing test substances in view of frequent false-negative reactions from petrolatum. It is recommended to use petrolatum for betalactams, carbamazapine, celecoxib, and other NSAIDs, water for acyclovir, gancyclovir, or alcohol for corticosteroids and steroid hormones to avoid false-negative results. [11],[17],[20],[21] It will be appropriate to test using all the vehicles as more validation is needed for optimal vehicles and test concentrations.
Controls are important for high predictive value of positive results and to exclude irritant reactions. The test substances need to be tested in healthy control subjects before actual testing. Similarly, vehicle used in preparation of test substances is also included for patch testing for the same reasons.
Time interval between adverse drug reaction and patch test
Although, there is some consensus of opinion that skin prick tests and intradermal tests should be performed with the offending drug after resolution of clinical signs and symptoms, clearance of the drugs used for the treatment of the adverse reaction, the offending drug or its metabolites from blood circulation or at least 3 weeks later, there exists some difference of opinion to carry out patch testing to identify the incriminating drug. The European Network on Drug Allergy recommends drug patch testing between 3 weeks and 3 months and according to European Society of Contact Dermatitis it is best to test at 6 weeks to 6 months after the complete resolution of clinical signs and symptoms. [22] As it is not known whether positive results will persist or last longer, it is advisable to perform drug patch test within 6 months after cutaneous adverse drug reaction has subsided completely and the patient is off any kind of treatment that may interfere with the results. [20]
Clinical Relevance
The overall sensitivity of drug patch test is low between 11% and 38% and the clinical usefulness varies across studies depending upon putative drug(s) and clinical features of the cutaneous adverse drug reaction. [8],[22] Although positive reactions have high predictive value, false-positive reactions are not uncommon. They may be due to IRs, which are frequent with drugs like sodium valproate even in 1% concentration [14] or from cross reaction between drugs themselves, within their own metabolites, or the metabolite of one drug and that of another. Cross reactivity is frequent especially among aromatic anti-convulsants (carbamazapine, phenytoin, phenobarbitone), lamotrigine and valproic acid, [23] among betalactum antibiotics, [8] acyclovir, valaciclovir and famcyclovir, [17],[24] and oxicams. [25]
False-negative drug patch test reactions are not uncommon especially in patient having non-immune drug reactions, when patch test drug has poor percutaneous absorption, e.g. allopurinol, [18] in the absence of concomitant factors like drug-virus (human herpes virus 6, HIV) interactions or the metabolite responsible for eliciting T-cell-dependent adverse reaction is not formed in the skin. [3] Wrong selection of vehicle, inadequate drug concentration and exposure time, and inappropriate timing for drug patch test are some of other possible reasons for false-negative reactions.
While specificity and negative predictive value of drug patch test is not yet determined, false-positive results need to be considered for final interpretation of the results. Predictive value of negative reactions is low and will not automatically exclude the offending drug. Barbaud et al. [11] observed 58% positive results on intradermal drug test among 60 patients with cutaneous adverse drug reactions having negative patch test with the suspected drug. It suggests that skin prick test and/or intradermal test will be useful when patch testing is negative. They also recommend immediate reading in urticaria-like drug reactions and delayed reading in other cutaneous drug reactions for accuracy.
Comments
Patch testing should preferably be performed on a previously involved skin or else a false-negative result is likely. Similarly, some locations may be inappropriate for patch testing and discretion must be practiced. Drug patch tests can be particularly helpful in determining the culprit drug in eczematous drug reaction, systemic contact dermatitis, maculopapular drug rash, DRESS, FDE and sometimes in SJS-TEN. It is easy to perform and any commercial drug can be used for testing (cf intradermal/prick tests). Adverse reactions to drug patch test are rare and it has been used safely in children as well. [26] However, possible precipitation of acute drug reaction, sensitization for cross reacting drugs, and possibility of contact allergy are some of the issues of ethical importance. Sometimes, drug patch test reactions occur earlier than 2 days (e.g., after 24 h in abacavir), or as late as 6-7 days after testing as in case of glucocorticosteroids or betalactam antibiotics. [8] Additional readings of results are highly desirable in such cases. In cases of negative patch test results, oral provocation test is considered to be the only reliable method to identify the culprit drug as in fixed drug eruption. In such a situation, oral provocation is performed first with the least implicated drug followed by more likely causes. A refractory period is also known especially in cases of FDE and a delay before and between patch testing and oral provocation is recommended. Patch testing, alone or in combination with oral provocation test, have a legitimate place in identifying the drug responsible for adverse reaction, especially, when multiple drugs are used to check for cross-sensitivities to medications or to generate a list of safe drugs.
| 1. |
Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000;356:1255-9.
[Google Scholar]
|
| 2. |
Balachandran C, Shenoi SD, Sarkar D, Ravikumar BC. Patch tests in adverse cutaneous drug reaction. Indian J Dermatol Venereol Leprol 2002;68:13-5.
[Google Scholar]
|
| 3. |
Friedmann PS, Ardern-Jones M. Patch testing in drug allergy. Curr Opin Allergy Clin Immunol 2010;10:291-6.
[Google Scholar]
|
| 4. |
Klein CE, Trautmann A, Zillikens D, Bröcker EB. Patch testing in an unusual case of toxic epidermal necrolysis. Contact Dermatitis 1995;33:448-9.
[Google Scholar]
|
| 5. |
Barbaud A. Drug patch testing in systemic cutaneous drug allergy. Toxicology 2005;209:209-16.
[Google Scholar]
|
| 6. |
Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002;57:45-51.
[Google Scholar]
|
| 7. |
Andrade P, Brinca A, Gonçalo M. Patch testing in fixed drug eruptions - A 20-year review. Contact Dermatitis 2011;65:195-201.
[Google Scholar]
|
| 8. |
Romano A, Viola M, Mondino C, Pettinato R, Di Fonso M, Papa G, et al. Diagnosing nonimmediate reactions to penicillins by in vivo tests. Int Arch Allergy Immunol 2002;129:169-74.
[Google Scholar]
|
| 9. |
Fischer T, Maibach HI. Patch testing in allergic contact dermatitis: An update. Sem Dermatol 1986;5:214-24.
[Google Scholar]
|
| 10. |
Wolkenstein P, Chosidow O, Fléchet ML, Robbiola O, Paul M, Dumé L, et al. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis 1996;35:234-6.
[Google Scholar]
|
| 11. |
Barbaud A, Reichert-Penetrat S, Tréchot P, Jacquin-Petit MA, Ehlinger A, Noirez V, et al. The use of skin testing in the investigation of cutaneous adverse drug reactions. Br J Dermatol 1998;139:49-58.
[Google Scholar]
|
| 12. |
Santiago F, Gonçalo M, Vieira R, Coelho S, Figueiredo A. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS). Contact Dermatitis 2010;62:47-53.
[Google Scholar]
|
| 13. |
Grandhe NP, Kaur I, Parsad D, Dogra S. Role of patch testing in antimicrobial drug eruptions. Contact Dermatitis 2004;50:259-61.
[Google Scholar]
|
| 14. |
Vatve M, Sharma VK, Sawhney I, Kumar B. Evaluation of patch test in identification of causative agent in drug rashes due to antiepileptics. Indian J Dermatol Venereol Leprol 2000;66:132-5.
[Google Scholar]
|
| 15. |
Gebhardt M, Wollina U. Allergy testing in serious cutaneous drug reactions - Harmful or beneficial? Contact Dermatitis 1997;37:282-5.
[Google Scholar]
|
| 16. |
Jindal N, Sharma NL, Mahajan VK, Shanker V, Tegta GR, Verma GK. Evaluation of photopatch test allergens for Indian patients of photodermatitis: Preliminary results. Indian J Dermatol Venereol Leprol 2011;77:148-55.
[Google Scholar]
|
| 17. |
Lammintausta K, Mäkelä L, Kalimo K. Rapid systemic valaciclovir reaction subsequent to aciclovir contact allergy. Contact Dermatitis 2001;45:181.
[Google Scholar]
|
| 18. |
Hamanaka H, Mizutani H, Nouchi N, Shimizu Y, Shimizu M. Allopurinol hypersensitivity syndrome: Hypersensitivity to oxypurinol but not allopurinol. Clin Exp Dermatol 1998;23:32-4.
[Google Scholar]
|
| 19. |
Barbaud A. Skin testing in delayed reactions to drugs. Immunol Allergy Clin North Am 2009;29:517-35.
[Google Scholar]
|
| 20. |
Barbaud A, Gonçalo M, Bruynzeel D, Bircher A, European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis 2001;45:321-8.
[Google Scholar]
|
| 21. |
Schuster J, Fabri M, Eming S, Hunzelmann N. Allergic drug eruption secondary to intravenous acyclovir. Acta Derm Venereol 2008;88:196-7.
[Google Scholar]
|
| 22. |
Gonçalo M, Oliveira HS, Monteiro C, Clerins I, Figueiredo A. Allergic and systemic contact dermatitis from estradiol. Contact Dermatitis 1999;40:58-9.
[Google Scholar]
|
| 23. |
Romano A, Viola M, Gaeta F, Rumi G, Maggioletti M. Patch testing in non-immediate drug eruptions. Allergy Asthma Clin Immunol 2008;4:66-74.
[Google Scholar]
|
| 24. |
Mansur AT, Pekcan Yaºar S, Göktay F. Anticonvulsant hypersensitivity syndrome: Clinical and laboratory features. Int J Dermatol 2008;47:1184-9.
[Google Scholar]
|
| 25. |
Gonçalo M, Figueiredo A. Cross reactions among oxicams in cutaneous adverse drug reactions. Contact Dermatitis 2002;46:S31.
[Google Scholar]
|
| 26. |
Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, Yavuz ST, Tuncer A, Kara A, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8-year-old girl. Australas J Dermatol 2012;53:274-7.
[Google Scholar]
|
Fulltext Views
9,005
PDF downloads
4,514





