Translate this page into:
Systemic skin whitening/lightening agents: What is the evidence?
Correspondence Address:
Devinder M Thappa
Department of Dermatology and STD, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry - 605 006
India
| How to cite this article: Malathi M, Thappa DM. Systemic skin whitening/lightening agents: What is the evidence?. Indian J Dermatol Venereol Leprol 2013;79:842-846 |
Introduction
The human skin color is one of the most perceptible phenotypic variations among humans and is determined primarily by the type and amount of melanin synthesized within melanosomes and the pattern of melanosome distribution within the melanocytes. Getting a lighter skin tone always draws a lot of interest, as for centuries, fair or light skin color has been a symbol of prominence, superiority and higher social ranking. In India, attitudes towards skin color have developed over more than 2000 years and reflect considerations of class and caste. Women especially Asian women are obsessed with fair skin and they would go to the ends of the earth to lighten their skin color as in most cases their marriage prospects or career opportunities are dominated by the hue of their skin. Hence, skin whitening products are a half a billion dollar industry today capitalizing on the insecurity of these individuals and currently, skin lightening is one of the most common forms of potentially harmful body modification practices in the world.
A host of skin lightening agents are available in dermatology and cosmetic market and newer agents are continuing to be introduced. A lot is known about topical skin whitening agents, but systemic skin whitening agents which are slowly gaining popularity do not have much evidence to their credit in the scientific literature. In this article, we have addressed the issues related to the use of these systemic skin whitening agents.
An overview of the pathway of melanin synthesis and the site of action of the most commonly used systemic skin whitening agent (glutathione [GSH]) is depicted in [Figure - 1]. The mechanisms governing pheomelanin to eumelanin balance are dependent on L-cysteine, GSH and tyrosinase related protein expression. Thus, as observed in the figure, the switching from eumelanogenesis to pheomelanogenesis can be influenced by modifying the ratio between cysteine and GSH levels. Pheomelanogenesis preferentially proceeds under conditions of high cysteine concentrations and low tyrosinase activity. [1]
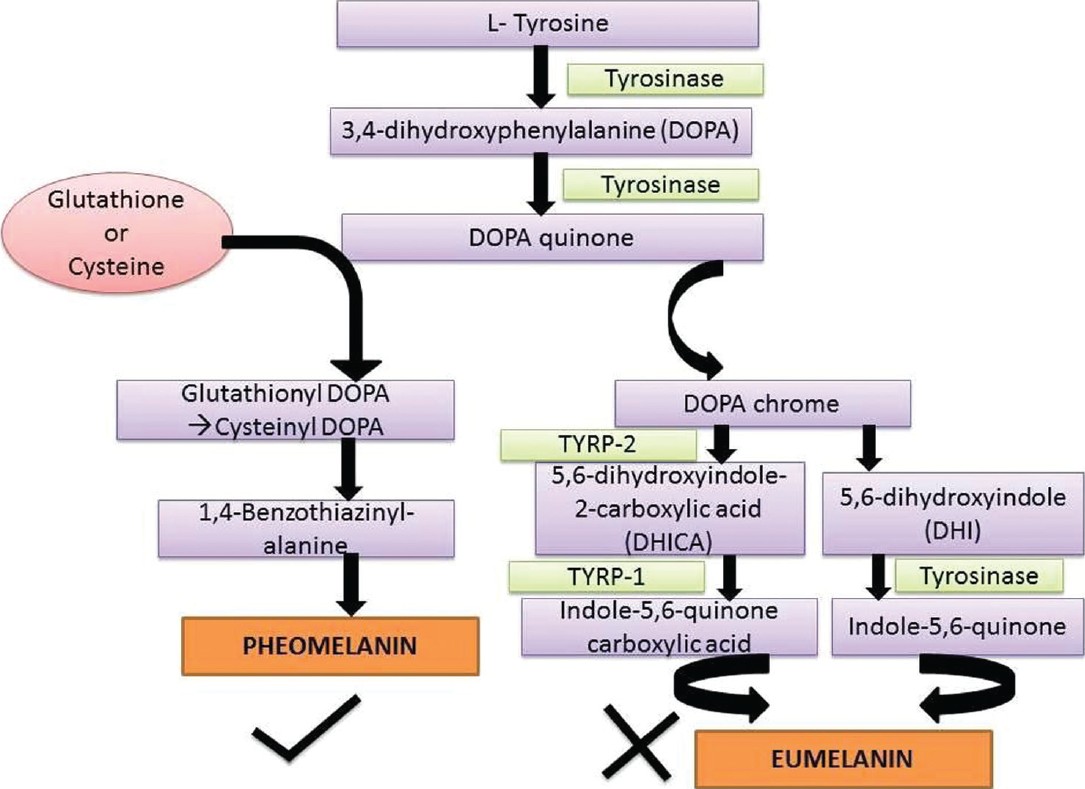 |
| Figure 1: The pathway of melanin synthesis and the site of action of glutathione and cysteine, shifting the balance towards pheomelanin synthesis |
Systemic Skin Whitening Agents Available in the Market
GSH
The most commonly used systemic skin whitening agent is GSH used alone or in various combinations, both as oral and intravenous formulations. GSH is an antioxidant synthesized in all mammalian cells from three amino acids-glutamate, cysteine, and glycine. It is also available naturally in watermelon, avocado, broccoli, spinach and tomatoes. GSH exists in cells mostly in the reduced form (GSH) which is constantly oxidized forming oxidized GSH and its supply is replenished by the action of GSH reductase. GSH is involved in various biochemical processes especially those involving scavenging of free radicals and detoxification of toxic compounds, it acts as coenzyme and helps in the transport of amino acids across the cell membranes. [1] Lower GSH levels are implicated in many diseases and GSH has been tried in the management of Alzheimer′s disease, Parkinson′s disease, multiple sclerosis, alcoholic hepatitis, atherosclerosis, acquired immunodeficiency syndrome, and chronic fatigue syndrome. It has been commonly used to combat the neuro- and nephrotoxicity associated with cisplatin chemotherapy [2] and this is the only Food and Drug Administration (FDA) approved indication for the intravenous form of GSH. The role of GSH as a skin whitening agent was an accidental discovery when skin whitening was noticed as a side effect of large doses of GSH. This led to extensive studies establishing its role in melanogenesis and the recent use of GSH as systemic skin whitening agent.
GSH exerts its action as skin whitening agent at various levels of melanogenesis which include the following: [1],[3]
- Interference with cellular transport of tyrosinase
- Direct inactivation of the enzyme tyrosinase by binding with the copper-containing active site of the enzyme
- Mediating the switch mechanism from eumelanin to phaeomelanin production as GSH is the major physiologic reservoir of cysteine and increase in cysteine levels results in switching of eumelanogenesis to pheomelanogenesis [Figure - 1]
- Quenching of free radicals and peroxides that contribute to tyrosinase activation and melanin formation
- Modulation of depigmenting abilities of melanocytotoxic agents.
Oral GSH is in the "generally regarded as safe" category of FDA and is usually marketed as a food or dietary supplement and hence it does not certainly need to be FDA or Bureau of Food and Drug (BFAD) approved. There are no provisions in the law for FDA to approve dietary supplements for effectiveness before they reach the consumer. However, FDA has banned the use of intravenous form of GSH for skin whitening in view of commonly reported side effects like skin rashes, Stevens Johnson syndrome, toxic epidermal necrolysis, derangement in thyroid and renal function and severe abdominal pain. [6]
Till date there is only one study published in English literature assessing the safety and efficacy of oral GSH as a skin whitening agent. [3] It was a randomized, double-blind, two-arm, placebo-controlled study conducted on 60 medical students in Thailand. They found 500 mg/day orally administered GSH, for 4 weeks to cause significant skin whitening when compared to placebo and they observed no significant adverse events. However, there were many flaws in the study-plasma GSH levels were not measured (as bioavailability of oral GSH is low), limited study period of 4 weeks, no follow-up to determine when the skin melanin indices return to their baseline values, medical students were chosen (young, otherwise healthy population) and the study was conducted during their college time to ensure that sun exposures were minimal. Hence, the results may be applicable to only young, otherwise healthy Asian individuals.
The promoters of GSH promote it at a dose of 20 and 40 mg/kg/body weight/day which is divided into two doses with a maintenance dose of 500 mg/day. [7] They claim that gradual systemic effect will be seen 1-3 months in medium brown skin, in 3-6 months in dark brown skin, in 6-12 months in very dark skin and in 2 years are more in black skin. Injectable GSH is given at a dose of 900 mg weekly by intravenous or intramuscular method and the sessions can be repeated 2-3 times a week. They claim skin whitening to occur as early as 2-3 weeks. [7]
GSH is also combined with many other agents like vitamin C to increase its absorption, N-acetyl cysteine to boost its level, alpha lipoic acid and other antioxidants like vitamin E and grape seed extract. Some oral preparations have dangerous combinations like monobenzone which causes irreversible depigmentation and hydroquinone which is banned by FDA as a carcinogen. [8]
L-cysteine peptide
Another agent promoted for skin whitening is L-cysteine peptide, which is claimed to be 3-5 times more potent than GSH and is BFAD approved. [9] Natural sources of L-cysteine include poultry, yogurt, egg yolks, red peppers, garlic, onions, broccoli, Brussel sprouts, oats, and wheat germ. L-cysteine along with L-glutamic acid and glycine is the rate-limiting precursor in the synthesis of GSH peroxidase and has been found that high concentrations of L-cysteine reduced the tyrosinase activity and produced more pheomelanin by way of cysteinyl dihydroxyphenylalanine (DOPA), the building block of pheomelanin [Figure - 1]. The cysteinyl DOPAs can be formed in two ways-directly by nucleophilic addition of cysteine to DOPA quinone or indirectly from GSH DOPA by action of gamma-glutamyl transferase and peptidase. [10],[11],[12] Hence L-cysteine peptide is promoted as skin whitening agent but without much scientific evidence to support its use for this indication.
Tranexemic acid
Tranexemic acid (trans-4-aminomethyl cyclohexane carboxylic acid), a plasmin inhibitor, commonly used as a haemostatic agent owing to its antifibrolytic action is also promoted as a systemic skin whitening agent especially as oral or intradermal injections for melasma. The skin whitening effects of tranexamic acid was incidentally found when it was used in the treatment of aneurysmal subarachnoid hemorrhage. It is a synthetic derivative of lysine and its therapeutic role in melasma was first studied by Nijor as early as in 1979, but only limited data exist in the literature regarding its use in melasma. Plasmin, is a protease that enhances the intracellular release of arachidonic acid, a precursor of prostanoid, and also elevates alpha-melanocyte stimulating hormone (α-MSH) processed from pro-opio-melanocortin. Both arachidonic acid and α-MSH can activate melanin synthesis by melanocytes. Tranexemic acid by way of its antiplasmin activity depletes the keratinocyte pool of arachidonic acid involved in ultraviolet (UV) induced melanogenesis. [13],[14],[15],[16],[17]
It has been used at a low dose of 250 mg twice a day for at least 3 months for the treatment of melasma and found to be effective. [15] But, it is not safe to use it for a long duration in view of its anti-hemorrhagic property resulting in side effects like venous thromboembolism, myocardial infarction, cerebrovascular accidents and pulmonary embolism. It is contraindicated in patients with acquired defective color vision, an active intravascular clotting condition, and hypersensitivity to tranexemic acid. [15] However, there is no scientific data available demonstrating the role of tranexemic acid as an overall skin whitening/lightening agent. It is even being promoted as intravenous injection for skin whitening at a dose of 500 mg every week for 1 or 2 months and 500 mg every month for maintenance. [18] Tranexamic acid has been found to produce good synergistic effect when combined with ascorbic acid or its derivatives and L-cysteine. [17]
Miscellaneous agents
Apart from these, large doses of vitamin C, hyaluronic acid, epidermal growth factor and combinations of multiple natural extracts (natural collagen extracts, bearberry extract, Glycyrrhiza glabra extract, Lycopene, Kelp, olive leaf extract, hawthorn, jujube, sea buckthorn, starch, coix seed, pearl extracts, etc.,) are also promoted for skin whitening in the form of food or dietary supplements with no scientific evidence.
Nevertheless, there are few animal studies and in vitro studies demonstrating the role of natural extracts like irradiated green tea polyphenol, [19] proanthocyanidin-rich extract from grape seeds, [20] ellagic acid-rich pomegranate extract, [21] and coumarin extracts from the plant Angelica dahurica, [22] in inhibiting melanogenesis resulting in skin whitening thereby recommending these agents as oral preparations for skin whitening. Procyanidins (pycnogenol, grape seed extracts) and Polypodium leucotomos have been found to effective and safe in the treatment of melasma and to prevent UV A induced pigmentary changes respectively. [23] But there are no trials demonstrating their efficacy in the treatment of post inflammatory pigmentation or in improving the general skin color.
The Scenario in India
In India, GSH is available as Dr. James GSH whitening pills [24] which contains 100% natural pure GSH with alpha T-acids costing 3500/- rupees for 60 capsules of 1000 mg, I-Fair tablets [25] which contains GSH in combination with vitamin C, vitamin E, grape seed extract, alpha lipoic acid, and glutanova 900 skin whitening injections [10] which contains GSH with vitamin C and collagen. These agents are being advertised in the internet and currently there are no market data available on the use of these agents by Indians. As mentioned in the introduction, most of Indian women′s marriage prospects are dominated by skin color paving way for irrational use of these agents by these women and may result in untoward effects. Hence, well conducted studies, establishing the role of these agents in skin whitening and documenting adverse events is the need of the hour.
Problems with Systemic Skin Whitening Agents
As already mentioned above, these agents are not FDA approved for skin whitening and no scientific evidence exists for their use. In addition, the injectable formulations are counterfeited and given by untrained people illegally and hence associated with the risk of sepsis, air embolism, transmission of human immunodeficiency virus, Hepatitis B, and use of non-sterile preparations that can lead to serious infections.
Another major question that remains unanswered as yet is that whether switching the normal machinery from eumelanin (which is protective against UV radiation) to pheomelanin (which photosensitizes UV-induced deoxyribonucleic acid damage as observed in cultured human melanocytes) by an external agent for long duration would result in an increased incidence of skin cancers. [26],[27]
As a general rule, the promoters of the systemic skin whitening agents list lactation, heart disease and hypersensitivity as contraindications for all these agents. Since there are not much human or animal studies advocating the role of these agents in skin whitening, there is no data on the relative and absolute contraindications, the appropriate dosing schedule and the long term side effects.
Economic Considerations
In India, the dermatology market is worth 1642 crore rupees ($ 410 million) and fairness-directed skin lightening cosmetic market (which are considered as ′′fast moving consumer goods′′) is 1000 crore ($ 250 million) resulting in a staggering 61% of the total dermatology market. [28] Companies manufacturing skin lightening products take advantage of the lax advertising laws and make unsubstantiated claims about their efficacy taking a horrendous toll on the consumers. The high-end skin whitening products are often labeled under the new quasi-pharmaceutical category called cosmeceuticals - a hybrid entity with pharmaceutical and cosmetic properties. This ambiguous labeling strategy allows promoters of high-end skin whitening and anti-aging products to make both pharmaceutical and cosmetic claims. The marketing trends are designed in such a way so as to blur the lines between cosmetics and food categories and food and pharmaceutical categories, thereby helping in evading the regulatory constraints for these products, eventually enticing more consumers to consume these relatively expensive and poorly regulated products. Thus, their claims can neither be independently verified, nor can potential risks to consumers be assessed. [29]
Conclusion
The desire for white and fair skin is a global phenomenon and it is being highly capitalized by both the cosmetic and dermatologic industries. It is an essential role of the dermatologist to make the public aware that skin lightening agents may progressively revert back the facultative color to the constitutive level and normally this color change will not go beyond the constitutive level. If such a change is claimed, it should be considered to be dangerous as such alterations can become non-reversible resulting in vitiligo and may also predispose to other complications like skin cancer as the normal biochemical processes are altered. Till date, systemic skin whitening agents do not have much scientific evidence regarding their use and strict laws should be enforced to ban the use of these agents until well conducted randomized controlled trials are available ensuring the safety and efficacy of these agents.
| 1. |
Villarama CD, Maibach HI. Glutathione as a depigmenting agent: An overview. Int J Cosmet Sci 2005;27:147-53.
[Google Scholar]
|
| 2. |
Hospers GA, Eisenhauer EA, de Vries EG. The sulfhydryl containing compounds WR-2721 and glutathione as radio- and chemoprotective agents. A review, indications for use and prospects. Br J Cancer 1999;80:629-38.
[Google Scholar]
|
| 3. |
Arjinpathana N, Asawanonda P. Glutathione as an oral whitening agent: A randomized, double-blind, placebo-controlled study. J Dermatolog Treat 2012;23:97-102.
[Google Scholar]
|
| 4. |
Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol 1992;43:667-9.
[Google Scholar]
|
| 5. |
Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, et al. Pharmacokinetics of glutathione and its metabolites in normal subjects. J Korean Med Sci 2005;20:721-6.
[Google Scholar]
|
| 6. |
Injectable glutha not FDA-approved. Available from: http://www.journal.com.ph/index.php/news/national/44605-injectable-glutha-not-fda-approved. [Last accessed on 2013 Apr 2].
[Google Scholar]
|
| 7. |
Skin whitening pills. Available from: http://www.magicproducts.in. [Last accessed on 2013 Apr 2].
[Google Scholar]
|
| 8. |
Levitt J. The safety of hydroquinone: A dermatologist′s response to the 2006 Federal Register. J Am Acad Dermatol 2007;57:854-72.
[Google Scholar]
|
| 9. |
Perfect white skin whitening and anti-aging tablets. Available from: http://www.perfectwhiteaim.blogspot.in. [Last accessed on 2013 Apr 2].
[Google Scholar]
|
| 10. |
Smit NP, Van der Meulen H, Koerten HK, Kolb RM, Mommaas AM, Lentjes EG, et al. Melanogenesis in cultured melanocytes can be substantially influenced by L-tyrosine and L-cysteine. J Invest Dermatol 1997;109:796-800.
[Google Scholar]
|
| 11. |
del Marmol V, Ito S, Bouchard B, Libert A, Wakamatsu K, Ghanem G, et al. Cysteine deprivation promotes eumelanogenesis in human melanoma cells. J Invest Dermatol 1996;107:698-702.
[Google Scholar]
|
| 12. |
Agrup G, Hansson C, Rorsman H, Rosengren E. The effect of cysteine on oxidation of tyrosine, DOPA, and cysteinyldopas. Arch Dermatol Res 1982;272:103-15.
[Google Scholar]
|
| 13. |
Ando H, Matsui MS, Ichihashi M. Quasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disorders. Int J Mol Sci 2010;11:2566-75.
[Google Scholar]
|
| 14. |
Tse TW, Hui E. Tranexamic acid: An important adjuvant in the treatment of melasma. J Cosmet Dermatol 2013;12:57-66.
[Google Scholar]
|
| 15. |
Karn D, S KC, Amatya A, Razouria EA, Timalsina M. Oral tranexamic acid for the treatment of melasma. Kathmandu Univ Med J (KUMJ) 2012;10:40-3.
[Google Scholar]
|
| 16. |
Wu S, Shi H, Wu H, Yan S, Guo J, Sun Y, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg 2012;36:964-70.
[Google Scholar]
|
| 17. |
Tranexemic acid. Available from: http://www.mcbiotec.com/products/?type=detail & id=33. [Last accessed on 2013 Jun 17].
[Google Scholar]
|
| 18. |
Beauty injections. Available from: http://www.beautyinjections.com/products/Tranexamic-Acid.html. [Last accessed on 2013 Jun 17].
[Google Scholar]
|
| 19. |
An BJ, Kwak JH, Son JH, Park JM, Lee JY, Park TS, et al. Physiological activity of irradiated green tea polyphenol on the human skin. Am J Chin Med 2005;33:535-46.
[Google Scholar]
|
| 20. |
Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, et al. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res 2003;16:629-38.
[Google Scholar]
|
| 21. |
Yoshimura M, Watanabe Y, Kasai K, Yamakoshi J, Koga T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci Biotechnol Biochem 2005;69:2368-73.
[Google Scholar]
|
| 22. |
Cho YH, Kim JH, Park SM, Lee BC, Pyo HB, Park HD. New cosmetic agents for skin whitening from Angelica dahurica. J Cosmet Sci 2006;57:11-21.
[Google Scholar]
|
| 23. |
Konda S, Geria AN, Halder RM. New horizons in treating disorders of hyperpigmentation in skin of color. Semin Cutan Med Surg 2012;31:133-9.
[Google Scholar]
|
| 24. |
Skin whitening pills in India. Available from: http://www.drjamespills.blogspot.in/2013/01/glutanova-900-skin-whitening-injections.html. [Last accessed on 2013 Apr 28].
[Google Scholar]
|
| 25. |
I-Fair-Glutathione supplement with vitamin C for skin whitening. Available from: http://www.cosmonova.in/i_fair.html. [Last accessed on 2013 Apr 28].
[Google Scholar]
|
| 26. |
Kvam E, Dahle J. Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage. J Invest Dermatol 2003;121:564-9.
[Google Scholar]
|
| 27. |
Wenczl E, Van der Schans GP, Roza L, Kolb RM, Timmerman AJ, Smit NP, et al. (Pheo) melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J Invest Dermatol 1998;111:678-82.
[Google Scholar]
|
| 28. |
Verma SB. Obsession with light skin - Shedding some light on use of skin lightening products in India. Int J Dermatol 2010;49:464-5.
[Google Scholar]
|
| 29. |
Mire A. The scientification of skin whitening and the entrepreneurial university-linked corporate scientific officer. Can J Sci Math Technol Educ 2012;12:272-9.
[Google Scholar]
|
Fulltext Views
23,253
PDF downloads
4,420





