Translate this page into:
A case of granular cell tumor with an interesting clinical course
2 Department of General Surgery, Uludag University, Bursa, Turkey
3 Department of Pathology, Uludag University, Bursa, Turkey
Correspondence Address:
Ozkan Kanat
Department of Medical Oncology, Uludag University, Gorukle, 16059, Bursa
Turkey
| How to cite this article: Kanat O, Ozguc H, Yalcinkaya U, Cubukcu E. A case of granular cell tumor with an interesting clinical course. Indian J Dermatol Venereol Leprol 2012;78:193-195 |
Sir,
A 45-year-old man was evaluated for a single, firm, painless and mobile cutaneous nodule 2 cm in size on the lateral aspect of his right upper arm. Total excision of the lesion was performed and findings on histopathological examination were consistent with typical benign granular cell tumor (GCT). The tumor cells were nonimmunoreactive for epithelial, muscle, endothelial and glial cell markers. Therefore, other diseases showing a clinical and histological resemblance to benign GCT including schwannoma, neurofibroma, rhabdomyoma, hibernoma, granular cell variants of basal cell carcinoma, melanoma and cutaneous sarcoma were excluded. Eight months later, a new small skin nodule appeared over the left scapular region. Total excision of the nodule was performed. The histopathological findings were similar to those of the first GCT. The patient failed to return for routine follow-up visits after the excision. Four years after the initial diagnosis, he presented again with large, firm and gradually enlarging subcutaneous nodules on the trunk, left antecubital fossa and left buttock [Figure - 1]. He also had anemia, with hemoglobin level 10 g/dl. A nodule was surgically removed from the left buttock, and histopathological examination revealed a GCT showing cytological features of malignant behavior. The tumor was found to comprise of large polygonal to spindle-shaped cells with an abundant eosinophilic granular cytoplasm and pleomorphic vesicular nuclei with prominent nucleoli [Figure - 2]. Intracellular PAS-positive globules were observed. Intense cellular pleomorphism and polygonal anaplastic cells dispersed between polymorphic giant cells were present. Three atypical mitoses were counted per 10 high-power fields. There were small foci of necrosis. Immunohistochemically, the tumor cells were positive for neuron-specific enolase, vimentin and S-100, but negative for cytokeratin 7, cytokeratin 20, CD68, HMB-45, CD1a, epithelial membrane antigen and myogenic differentiation-1 [Figure - 3]. More than 60% of the tumor cells were positive for Ki-67. Computed tomography scan of the abdomen and chest was planned on an outpatient basis. But, he presented with massive upper gastrointestinal tract bleeding. Endoscopy revealed a solitary bleeding tumor-like lesion in the stomach and he underwent emergency gastrectomy for uncontrolled bleeding. During laparotomy, a tumor mass was also found within the gallbladder, and cholecystectomy was performed. The liver appeared normal. On gross examination of the resected specimen, two large tumors were found in the midbody of the stomach. They measured 8.0 and 5.0 cm in diameter, respectively. These gastric tumors and gallbladder tumor were diagnosed as malignant GCT. Histopathological features of the tumors were similar to those seen in the tumor removed from the patient′s buttock. In the stomach, the tumors extended to the serosal surface. Several perigastric lymph nodes were also involved. In the gallbladder, the tumor invaded the serosa. Postoperative clinical course was uneventful. The nature of the disease and scarcity of treatment options was discussed with the patient. Systemic chemotherapy with cisplatin (60 mg/m 2 intravenously on day 1) plus capecitabine (1000 mg/m 2 orally twice daily on days 1 through 14) was started. The treatment was repeated every 3 weeks. Three months after the introduction of the chemotherapy, he developed multiple bone and brain metastases.
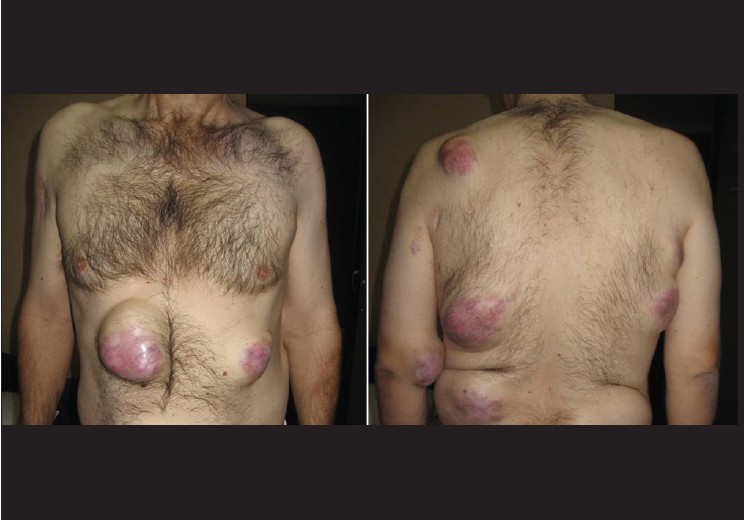 |
| Figure 1: Large and firm subcutaneous nodules over the trunk and left antecubital fossa |
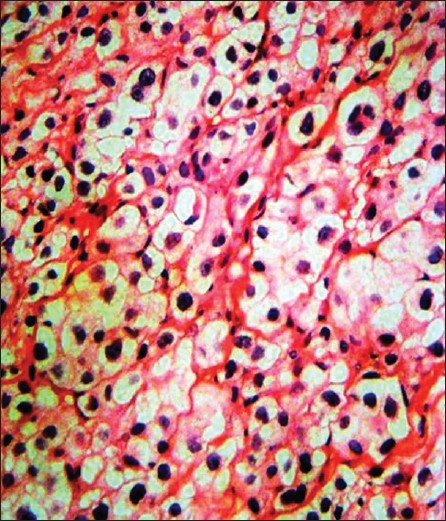 |
| Figure 2: A granular cell tumor is composed of sheets of polyhedral cells with delicate fibrovascular septa. Some nuclei are small and dark and the others are relatively large and appear vesicular with nuclear chromatin pattern (H and E, × 100) |
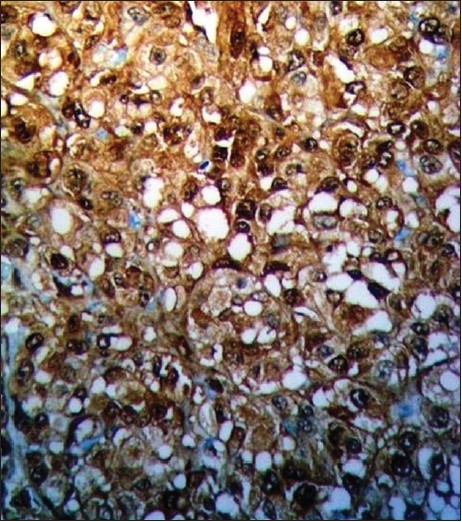 |
| Figure 3: Strong reactivity for S-100 protein is seen in granular cell tumor (S-100, × 200) |
GCT is a very rare soft tissue lesion originating from Schwann cells. [1] The dermis and the tongue are the most frequently involved organs. [2] The involvement of the gastrointestinal tract occurs in 5-9% of GCT cases, mainly in the esophagus. [3] Very rarely, however, multiple gastrointestinal GCTs can be detected in the same patient. [4] In most patients, this tumor shows benign behavior. However, metastatic spread may be seen in 1-2% of patients with GCT. Six histological features, including increased mitotic activity, necrosis, nuclear pleomorphism, spindling of tumor cells, increased nuclear to cytoplasmic ratio and vesicular nuclei with prominent nucleoli, have been found to be highly correlated with malignant clinical behavior for GCT. [5] None of these features, however, were observed in the patient′s original tumor. This unusual case history illustrates the variable clinical course of GCT, and gives some specific messages to clinicians. We still do not know enough about this tumor. Histological features of these tumors may not always predict their clinical behavior. In patients with benign cutaneous GCT, recurrences can occur many years after the original diagnosis. Recurrent disease can also involve gastrointestinal organs besides the skin, and represent malignant transformation of GCT. Therefore, the clinician should remain alert for signs and symptoms of gastrointestinal involvement in patients with GCT. Anemia may be the first sign of this unexpected clinical course, as in our case. We believe that documentation of patients who show an unexpected clinical course may help in better understanding of the nature and prognosis of this rare entity.
| 1. |
Ordonez NG. Granular cell tumor: A review and update. Adv Anat Pathol 1999;6:186-203.
[Google Scholar]
|
| 2. |
Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, et al. Granular cell tumor: A clinicopathologic study of 110 patients. J Surg Oncol 1980;13:301-16.
[Google Scholar]
|
| 3. |
Johnston J, Helwig HB. Granular cell tumors of the gastrointestinal tract and perianal region: A study of 74 cases. Dig Dis Sci 1981;26:807-16.
[Google Scholar]
|
| 4. |
Maekawa H, Maekawa T, Yabuki K, Sato K, Tamazaki Y, Kudo K, et al. Multiple esophagogastric granular cell tumors. J Gastroenterol 2003;38:776-80.
[Google Scholar]
|
| 5. |
Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: Diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol 1998;22:779-94.
[Google Scholar]
|
Fulltext Views
3,112
PDF downloads
2,303





