Translate this page into:
A clinical study of psoriatic arthropathy
2 Department of Orthopaedics, Rajah Muthiah Medical College, Annamalai University, Annamalai Nagar, Tamilnadu, India
Correspondence Address:
PVS Prasad
88, AUTA Nagar, Sivapuri post, Annamalai Nagar - 608 002, Tamilnadu
India
| How to cite this article: Prasad P, Bikku B, Kaviarasan P K, Senthilnathan A. A clinical study of psoriatic arthropathy. Indian J Dermatol Venereol Leprol 2007;73:166-170 |
Abstract
Background: The incidence of uncomplicated psoriasis is 1-3% in the general population. Arthritis is found in increased frequency in psoriatic patients and its incidence is estimated to be 5-7%. Aim: To assess the prevalence of arthritis in psoriatic patients. Methods: Four hundred and seventy-two psoriatic patients were enrolled in the study out of which 40 patients had (psoriatic) arthropathy (PsA). Severity of psoriasis was assessed by the psoriasis area and severity index (PASI). Routine blood investigations were carried out along with radiological investigations. Results: Forty percent of the 40 PsA patients were in the age group of 51-60 years. Seven patients out of the 40 (17.5%) psoriatic arthropathic (PsA) patients had a family history of psoriasis. Nail involvement was observed in 37 cases (92.5%). Rheumatoid factor was present in five out of the 40 (12.5%) PsA patients. Serum uric acid levels were above normal in eighteen out of the 40 (45%) PsA patients. Asymmetric oligoarthropathy was the most commonly observed feature in 42.5% of the 40 PsA patients. Narrowing of joint spaces and erosions were observed in 62.5% and 45% of the 40 PsA patients. Conclusion: There is an association between the duration of skin lesions and duration of arthropathy. Similarly the PASI score is also directly related with arthropathy.










 |
| Figure 3: Radiological changes in psoriatic arthritis |
 |
| Figure 3: Radiological changes in psoriatic arthritis |
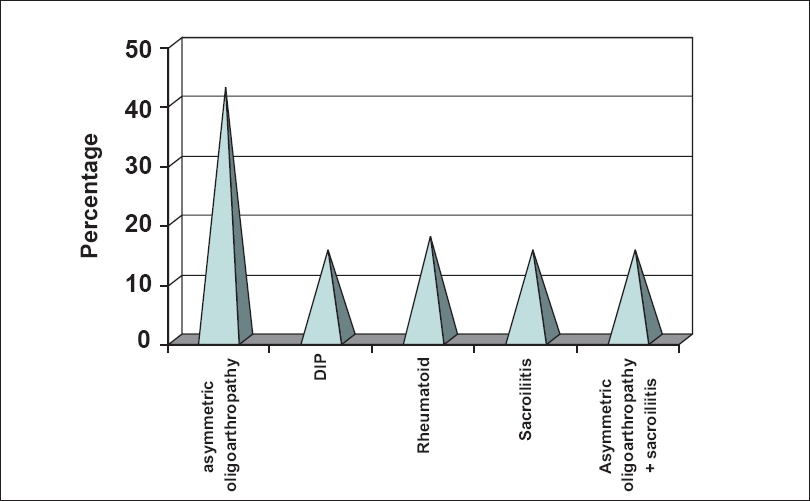 |
| Figure 2: Distribution types of psoriatic arthropathy |
 |
| Figure 2: Distribution types of psoriatic arthropathy |
 |
| Figure 1: Age distribution of psoriatic arthropathy |
 |
| Figure 1: Age distribution of psoriatic arthropathy |
Introduction
The recognition of psoriatic arthropathy (PsA) as a separate entity is a relatively recent occurrence. However, Jean Louis Alibert, a French physician, described an association between psoriasis and arthritis in 1818 [1] and Earnest Bazin and Charles Bourdillon conducted further studies on this condition. [2] In 1973, Moll and Wright identified and related clinical aspects which affected both the joints and the skin, defining PsA as an inflammatory arthritis, generally with a negative rheumatoid factor and associated with psoriasis. [2] The true prevalence of psoriasis and PsA is not known with certainty, partly because of the elusive nature of the psoriatic skin lesion. The prevalence of uncomplicated psoriasis is between 1-3% in the general population. Arthritis is found in increased frequency in psoriatic patients and its incidence is estimated to be 5-7%. In severe psoriasis, arthritis can occur in up to 30-40% of the patients. [3] As there are very few Indian studies on PsA, [4] we undertook this clinical study to assess the prevalence of PsA in psoriatic patients.
Methods
The study was conducted in the Department of Dermatology, Venereology and Leprosy at a rural hospital in Tamil Nadu for one year. Forty psoriatic patients with PsA attending the outpatient department were included in the study. Details of demographic factors, history, clinical features and type of psoriasis were all recorded. The severity of the disease and the extent of involvement were assessed by using the psoriasis area severity index (PASI). Routine investigations including those for rheumatoid factor, serum uric acid, Venereal Disease Research Laboratory (VDRL) and enzyme-linked immuno-sorbent assay (ELISA) for human immunodeficiency virus (HIV) were done for all patients. Appropriate radiological investigations were carried out in all cases. X-rays were reported both by a radiologist and an orthopedic surgeon. The results were analyzed by using the Chi-square test. The study was conducted after obtaining permission and informed consent from the ethical committee of the institution and all patients respectively.
Results
Out of 21850 patients who attended the dermatology outpatient department of our hospital during the study period of one year, there were 472 new cases of psoriasis (2.16%). Forty out of the 472 patients suffered from PsA (8.47%). The age distribution of PsA is shown in [Figure - 1]. Sixteen out of 40 PsA patients were in the age group of 51-60 years whereas five patients belonged to the 31-40 years age group. The oldest patient was 68 years while the youngest was 34 years of age. The PsA study group comprised 34 males (85%) and six females with a male to female ratio of 5.6:1.
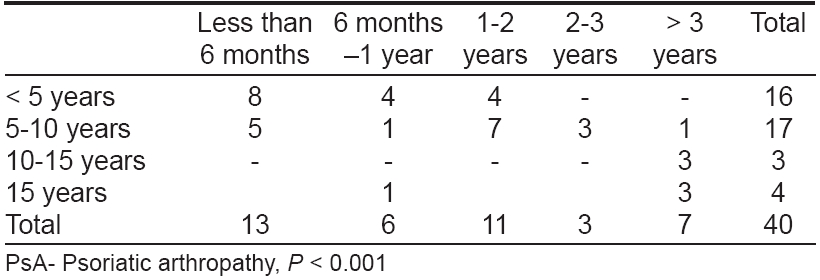
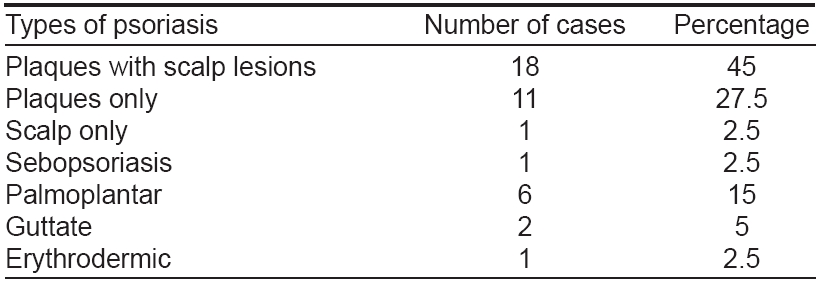
The duration of skin disease in patients with PsA is shown in [Table - 1]. Out of forty PsA patients, 42.5% had psoriatic skin lesions for 5-10 years and 40% for < 5 years. Thirteen patients (32.5%) had noticed symptoms of PsA of < six months duration [Table - 2]. Family history of psoriasis was observed in seven out of 40 PsA patients (17.5%). The clinical type of psoriasis correlated with PsA is shown in [Table - 3]. Chronic plaque psoriasis with scalp psoriasis was seen in 18 patients while chronic plaque psoriasis without scalp involvement was noticed in another 11 patients. Eight PsA patients (20%) showed K φbner′s phenomenon and a positive Auspitz sign was seen in 33 patients. Various types of nail changes were observed and are shown in [Table - 4].
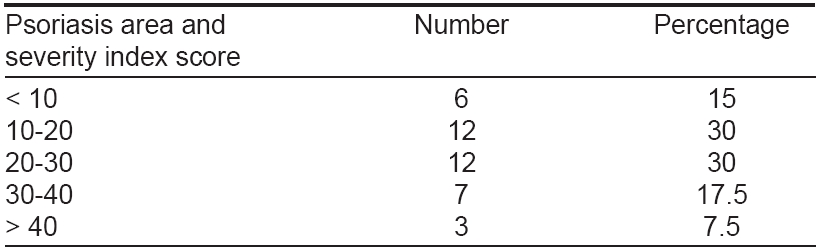
The nail changes ranged from pitting, ridging, subungual hyperkeratosis, onycholysis, dystrophy and discoloration. Among these, pitting was the most common nail change observed in 29 patients (72.5%). PASI score in patients with PsA is shown in [Table - 5]. The score ranged from 6.2 to 42.4 with a mean of 17.8 and standard deviation (SD) of 4.8. Asymmetric oligoarthropathy was seen in 17/40 patients (42.5%). In five patients, there was an overlap between asymmetric oligoarthropathy and spondylitis and in another two, both asymmetric oligoarthropathy and sacroiliitis were seen [Table - 6].
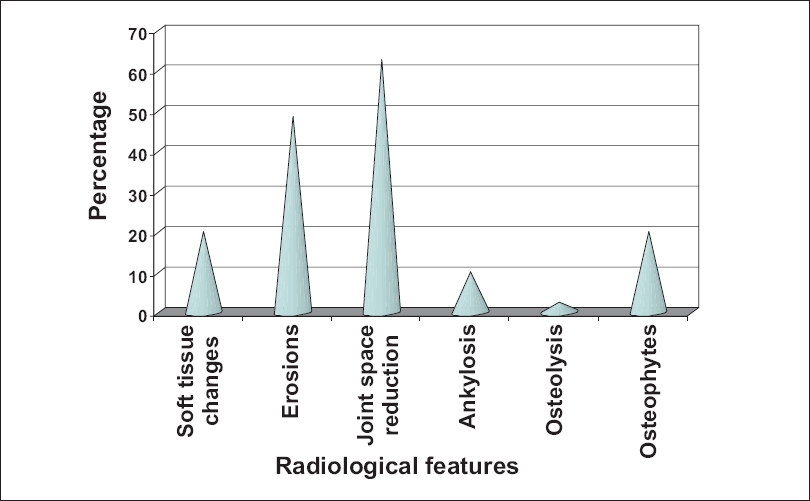
Investigations revealed positive rheumatoid factor test results in five patients (12.5%). The serum uric acid level was above normal limits in 18 patients (45%). It ranged from 2.8-8.1 mg/dl with a mean of 6.21 and SD of 1.44. Erythrocyte sedimentation ratio (ESR) was raised in 21 patients (52.5%). On radiological examination, 62.5% showed narrowing of joint spaces and 45% showed erosions. The other changes are shown in [Figure - 2]. Out of the 40 PsA patients in the study group, 82.5% had psoriatic skin lesions for < 10 years, while in 30 patients (75%), the duration of arthropathy was < 2 years. Association between the duration of skin lesions and duration of PsA is shown in [Figure - 3]. Similarly, PASI score in relation to type of PsA is shown in [Table - 7].
Discussion
Prevalence of PsA in our study period is found to be 8.47%. Prevalence of PsA in the general population could not be determined, but according to Braun-Falco, it lies between 0.02 and 0.1%. [3] Moll estimated the prevalence of PsA among psoriatics to be 5-7%. [5] However, the prevalence rates are variable and may be as high as 30-40% in psoriatic patients with severe skin manifestations. The age of onset varies from 30-50 years, a range which is similar to that of rheumatoid arthritis. In our study, 40% of patients were in the age group of 51-60 years. PsA is found most commonly in the age group of 41-60 years and its incidence decreases towards the outer limits of the range.
Rajendran from Chennai also found a peak incidence (69%) of PsA in the 4 th and 5 th decades of life. [4] Wright reported an equal incidence of PsA in both sexes. We found a male preponderance which could also be due to the overall proportion of male patients visiting the clinic being higher. Psoriatic skin lesions preceded PsA in 39 patients (97.5%), while in one patient, they occurred simultaneously. Similar observations were made by earlier authors. [6] We also found that PsA is relatively rare after ten years of onset of skin lesions. Almost all patients in this group were taking some disease-modifying agents, which could be the reason for the lower prevalence in those with duration of skin lesions exceeding ten years.
All patients who were in the fourth decade of life who developed psoriasis, also developed PsA within twelve months. Family history of psoriasis was found in 27.5% of patients in our study. Recent genetic studies suggest that certain HLA antigens like B13, B17, B18, B27, DR4 and DR7 were found more commonly in patients with PsA than in patients with only psoriatic lesions. [7],[8] According to Finzi, heredity is a predisposing factor in PsA. [2] Chronic plaque type of psoriasis was the most common presentation with scalp involvement in most of the cases. Rajendran also observed chronic plaque type in 81% of the patients and opined that the scalp was a hidden site. [4]
Kobner′s phenomenon was observed in 20% of our patients. On the basis of K φbner′s phenomenon of the skin, Moll et al. hypothesized that repeated micro or macro traumas may have a profound ′Koebner effect′ on the joints. [9] A positive Auspitz sign was seen in 82.5% of the patients. This indicated that the disease is still active in such patients. Nail changes were observed in 92.5% of cases while 65% showed more than one nail finding. A similar association has been noticed in the earlier studies also. [10] In some studies, the observation of nail changes in PsA patients was considered secondary to arthritis of underlying joints. [11],[12] Distal interphalangeal (DIP) arthropathy was commonly associated with onycholysis and dystrophic nail changes in seven patients.
We could not correlate the type of nail change with the type of psoriasis as the sample size was small and various morphological types of nail changes were seen in a single patient. Although PsA is a sero-negative arthritis and the absence of rheumatoid factor is a characteristic laboratory finding, it may be present in 3% of PsA patients, which is the same as its incidence in the general population. [3] We have found 12.5% of cases testing positive for rheumatoid factor. Two of these patients had symmetric rheumatoid type of arthropathy with abnormally raised ESR. These cases create a diagnostic difficulty except for the presence of typical psoriatic lesions.
Serum uric acid levels were found to be elevated in a number of studies. This is due to increased purine metabolism due to high cell turnover. Slightly elevated levels do not usually end up in gout. Our results were consistent with those of Lambert and Wright who found a high prevalence of serum uric acid values above normal, but a mean value inside the normal range. [12] Hyperuricemia was noted in 41.6% of the cases by Nigam in a study on psoriatic arthritis, indicating extensive involvement of skin. [13] ESR values were raised in 52.5% of cases in the study group. ESR is widely used as a nonspecific marker of joint inflammation to assess disease activity. The other laboratory indicators are a-2 globulin, b globulin and C-reactive protein, whose levels may also be elevated. [14] We could not conduct these tests due to the lack of necessary facilities.
Previous studies did not measure PASI scores whereas we found a direct correlation between a high PASI and various types of PsA. Four out of six patients with DIP involvement alone had a PASI of < 10. This shows that less severe skin involvement is associated with minimal joint involvement. Association of elevated PASI scores in other types of PsA is statistically significant. However, we could not relate any specific type of joint involvement with the severity of the skin disease. PsA is considered a sero-negative spondyloarthropathy, a group of diseases that includes ankylosing spondylitis, reactive arthritis and Reiter′s syndrome and the arthritis associated with inflammatory bowel disease. These diseases share a predilection for asymmetric peripheral arthritis and axial or spinal involvement.
As with other spondyloarthropathies, musculoskeletal manifestations of PsA may also include inflammation at the site attachment of tendons and ligaments (enthesitis), especially at the site of the Achilles tendon insertion and the insertion of the plantar fascia into the calcaneus. Dactylitis, also described as a sausage digit, may be seen. These digits develop swelling and tenderness of the entire finger or toe because of inflammation of the tendons along with the involved joints. [15] Asymmetric oligoarthropathy was most commonly seen in our series, which was found in 42.5% cases. We did not encounter patients with arthritis mutilans in our series. Finzi also reported asymmetric oligoarthritis as the largest subgroup of PsA in 70% of the cases. [2]
The longer the duration of psoriasis, the more severe is the PsA. Brower suggested that the initial lesions of PsA are marginal erosions and the narrowing of joint spaces while osteolysis, ankylosis and new bone formation are seen in the more advanced stages of the disease. [15],[16] We also correlated the type and severity of radiologic changes and consequent damage to the joints affected with the duration of arthropathy. The most common radiologic changes observed in the study group were of joint space narrowing in 62.5% and erosions in 45% of the patients with many patients showing more than one type of change. Long-standing cases showed more damaging radiologic changes like ankylosis (7.5%) and early osteolysis (5%) and all these patients had psoriasis of > 3 years duration.
Seven patients showed only soft tissue changes with no obvious bony changes in the X-rays and all of them presented within the first six months of onset of arthropathic symptoms. Although PsA has traditionally been viewed as a disease with a benign prognosis, radiographic evidence, as described previously, indicates that the disease is more progressive and destructive than what was previously thought. Although the highest rate of peripheral joint involvement in PsA seems to be within the first 12 months of disease onset, the disease has been shown to be progressive in terms of number of joints affected and damage to those joints. It has been suggested that possible indicators of poor prognosis include younger age at onset, extensive skin involvement and certain HLA antigens. [17] From our study, we could conclude a direct correlation between severity of PsA and age of onset of the disease. Ongoing research into the epidemiology and outcomes of PsA can provide insight into the patient characteristics, such as multiple involved joints, that may be indicators of poor prognostic outcomes.
| 1. |
Moll JM. Psoriatic arthritis. In : Mier PD, Peter CM, Vande, editors. Text book of Psoriasis. 1 st ed. Churchill Livingstone: New York; 1986. p. 55-83.
[Google Scholar]
|
| 2. |
Finzi AF, Gibelli E. Psoriatic arthritis. Int J Dermatol 1991;30:1-7.
[Google Scholar]
|
| 3. |
Braun-Falco O, Ruzicka T. Psoriatic arthritis. Int J Dermatol 1994;33:320-2.
[Google Scholar]
|
| 4. |
Rajendran CP, Ledge SG, Rani KP, Mahadevan R. Psoriatic arthritis. JAPI 2003;51:1065-8.
[Google Scholar]
|
| 5. |
Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55-78.
[Google Scholar]
|
| 6. |
Winchester R. Psoriatic arthritis. Dermatol Clin 1995;13:779-92.
[Google Scholar]
|
| 7. |
Moll JM, Wright V. Familial occurrence of psoriatic arthritis. Ann Rheum Dis 1973;22:181-201.
[Google Scholar]
|
| 8. |
Gladman DD, Anhorn KA, Schachter RK, Mervart H. HLA antigens in psoriatic arthritis. J Rheumatol 1986;13:586-92.
[Google Scholar]
|
| 9. |
Espinoza LR, Cueller ML. Psoriatic arthritis: Management. In : Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 3 rd ed. Mosby: London; 1998. p. 1259-65.
[Google Scholar]
|
| 10. |
Espinoza LR, Cueller ML. Psoriatic arthritis: Management. In : Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 3 rd ed. Mosby: London; 1998. p. 1259-65.
[Google Scholar]
|
| 11. |
Scarpa R, Oriente P, Pucino A, Torella M, Vignone L, Riccio A, et al . Psoriatic arthritis in psoriatic patients. Br J Rheumatol 1984;23:246-50.
[Google Scholar]
|
| 12. |
Lambert JR, Wright V. Serum uric acid levels in psoriatic arthritis. Ann Rheuma Dis 1977;36:234-7.
[Google Scholar]
|
| 13. |
Nigam P, Singh DS, Matreja VS, Saxena HN. Psoriatic arthritis: A clinical radiological study. J Dermatol 1980;7:55-9.
[Google Scholar]
|
| 14. |
Gladman DD, Shukett R, Russel ML, Thorne JC, Schachter RK. Psoriatic arthritis: An analysis of 220 patients. Q J Med 1987;62:127-41.
[Google Scholar]
|
| 15. |
Ruderman EM, Tamber S. Psoriatic arthritis: Prevalence, diagnosis and review of therapy for the dermatologists. Dermatol Clin 2004;22:477-80.
[Google Scholar]
|
| 16. |
Brower AC, Allman RM. Pencil pointing: A vascular pattern of deossification. Radiographics 1983;3:315-8.
[Google Scholar]
|
| 17. |
Moll JM. The clinical spectrum of psoriatic arthritis. Clin Orthop 1979;143:66-75.
[Google Scholar]
|
Fulltext Views
2,933
PDF downloads
1,618





