Translate this page into:
A clinical study of skin changes in pregnancy
Correspondence Address:
Devinder Mohan Thappa
Department of Dermatology and STD, JIPMER, Pondicherry - 605 006
India
| How to cite this article: Kumari R, Jaisankar T J, Thappa DM. A clinical study of skin changes in pregnancy. Indian J Dermatol Venereol Leprol 2007;73:141 |
Abstract
Background: During pregnancy profound immunologic, metabolic, endocrine and vascular changes occur, that are responsible for the changes of the skin and its appendages, both physiologic and pathologic. Aims: We undertook a clinical study to find out the frequency and pattern of skin changes in pregnant women. Methods: All consecutive pregnant women were included in the study. Results: A total of 607 pregnant women were included in this study. Of these, 303(49.9%) pregnant women were primigravida and 304(51.1%) were multigravida. Skin changes grouped into: physiological changes (all cases), specific dermatoses (22 cases) and other dermatoses affected by pregnancy (125 cases). Most common physiological changes were pigmentary alterations seen in 555 (91.4%) followed by striae seen in 484(79.7%) cases. Of the various specific dermatoses of pregnancy, pruritic urticarial papules and plaques of pregnancy (PUPPP) was the most common disorder (14 cases) followed by pruritus gravidarum (5 cases). The most common dermatoses affected by pregnancy were candidal vaginitis (17 cases), acne vulgaris (15 cases), skin tags (15 cases), eczemas (14 cases). Conclusion: This study brings into focus various skin changes during pregnancy in south India.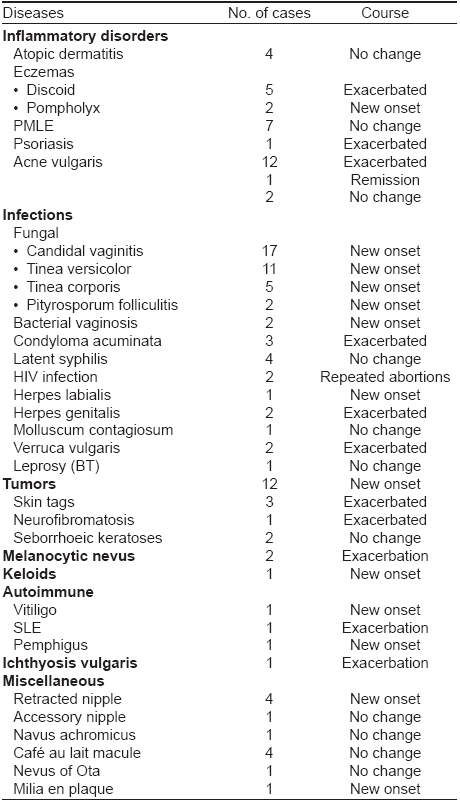



INTRODUCTION
During pregnancy profound immunologic, metabolic, endocrine and vascular changes occur, which make the pregnant woman susceptible to changes of the skin and appendages, both physiologic and pathologic. [1] These alterations may range from normal cutaneous changes that occur with almost all pregnancies, to common skin diseases that are not associated with pregnancy, to eruptions that appear to be specifically associated with pregnancy. Likewise, the concerns of the patient may range from cosmetic appearance, to the chance of recurrence of the particular problem during a subsequent pregnancy, to its potential effects on the fetus in terms of morbidity and mortality. [2] Moreover, pregnancy modifies the course of a number of preexisting dermatological conditions. [3] We undertook this study to know the frequency and pattern of skin changes in pregnant women and various clinical parameters affecting them in south India.
METHODS
The study was conducted in the out-patient department of Obstetrics and Gynecology, JIPMER, Pondicherry. Ethical committee clearance was obtained. All pregnant women seen between September 2003 and June 2005 were included in the study irrespective of the duration of pregnancy and gravidity. Informed consent was obtained before the interview and clinical examination. A total of 607 pregnant women were included in the study. Detailed history including demographic data, chief complaints related to skin, presence of itching, skin lesions, onset in relation to duration of pregnancy, jaundice, vaginal discharge, past or family history of similar lesions, exacerbating factors, associated medical or skin disorders etc. was elicited and recorded.
Complete cutaneous examination was done in all cases to study all the physiological changes of skin and its appendages. If any specific dermatosis of pregnancy was present, the morphology of skin lesions, distribution and the sites involved were studied. Relevant systemic examination was carried out. If any preexisting skin disease was present, any evidence of exacerbation or remission was recorded.
Appropriate investigations were done to confirm diagnosis if required. Bedside laboratory procedures like Tzanck smear, KOH mount and Gram′s stain were carried out. To confirm diagnosis skin biopsy and DIF were done in a few cases. In all cases with history of pruritus related to specific disorders of pregnancy, liver function tests were done. Screening with VDRL and ELISA for HIV was done in all the cases. Examination of the ′contact′ was done in all cases of sexually transmitted disease. Results were tabulated and analyzed.
RESULTS
A total of 607 pregnant women were recruited in our study from September 2003 to June 2005. Of these, 303 (49.9%) were primi gravidas and 304 (51.1%) were multi gravidas. Their age range was 18 to 36 years with a mean of 23 years. Most of them presented in the second (139 cases, 22.9%) or third trimester (444 cases, 73.1%). Most of them (597 cases, 98.4%) were native of South India and majority belonged to skin type IV (425 cases,70%) and V (182 cases,30%).
Pregnancy dermatoses were divided into three categories
- Physiological skin changes
- Specific dermatoses of pregnancy
- Skin diseases affected by pregnancy
Physiological changes were seen in all cases (607). Twenty two cases of specific dermatoses of pregnancy were seen. Other dermatoses affected by pregnancy were seen in 125 cases.
Majority of these pregnant women 546 (89.9%) had no skin related complaints. In those who had primary complaints, itching was the most common primary complaint (68, 11.2%) followed by complaints of presence of skin lesions (52, 8.6%), vaginal discharge (19, 3.1%), melasma (15, 2.5%), miliaria (10, 1.6%) and vulval growth (3, 0.5%).
Past history of striae and pigmentary changes was observed in 204 multi gravidas. None of them had used oral contraceptive pills. A history of extramarital sexual exposure was obtained in 2 pregnant women and six of their respective partners.
PHYSIOLOGICAL SKIN CHANGES
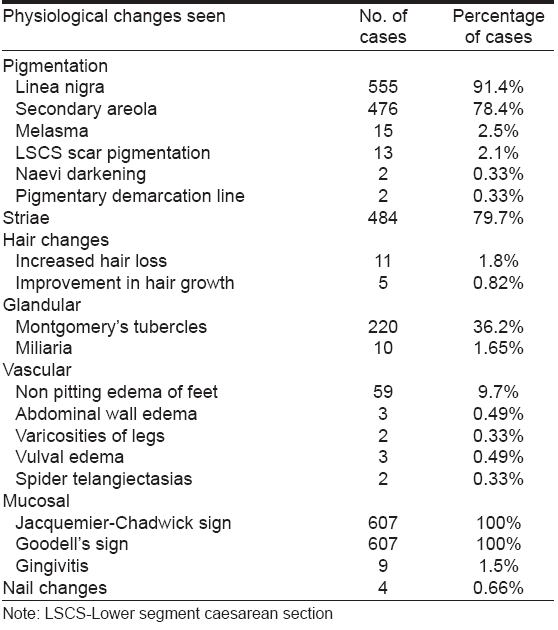
Among the physiological skin changes observed, most common were pigmentary changes in 555 (91.4%) cases including hyperpigmentation of skin, melasma, linea nigra, development of secondary areola followed by striae in 484 (79.7%) cases [Table - 1].
SPECIFIC DERMATOSES OF PREGNANCY
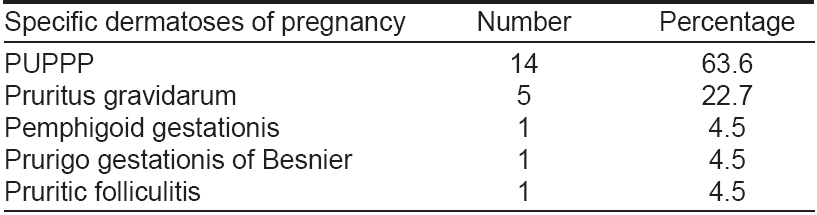
Out of 607 pregnant women seen during the study, 22 pregnant women had specific dermatoses of pregnancy [Table - 2].
Pruritic urticarial papules and plaques of pregnancy (PUPPP)
In this study, 14 pregnant women were found to have pruritic urticarial papules and plaques of pregnancy (PUPPP). Of these fourteen, 12 (85.7%) were primigravidas and 2 were multi gravidas (14.3%). One patient had fetal complications with IUGR and preterm delivery of a male infant weighing 2.2 kgs.
Pruritus gravidarum
Five cases of pruritus gravidarum were seen in this study. Of these five, three (60%) were primi gravidas and 2 were multi gravidas (40%). Liver function tests were normal except for raised alkaline phosphatase in 3 patients (60%). No adverse fetal outcome was seen in the five pregnant women.
DERMATOSES AFFECTED BY PREGNANCY [Table - 3]
Certain dermatoses like atopic dermatitis (4 cases), polymorphous light eruption (7 cases), latent syphilis (4 cases), molluscum contagiosum (one case), leprosy (one case), seborrheic keratoses (2 cases), retracted nipple (4 cases), Cafι au lait macule (4 cases), accessory nipple, nevus achromicus and nevus of Ota (one case each) did not show any change.
DISCUSSION
Many of the symptoms and signs are so common that they are not usually considered as being abnormal, but regarded as physiological and can sometimes provide contributory evidence of pregnancy. The commonly encountered physiological changes include striae distensae (occurring in up to 90% of pregnant women), hormonal alterations resulting in melasma (occurring in up to 75% of women during pregnancy) and generalized hyperpigmentation. Vascular alterations result in edema, palmar erythema, spider nevi, varicosities, cutis marmorata, gingival edema and redness. Some women also notice hair and nail changes. Similarly the activity of eccrine and sebaceous glands increases, while that of apocrine gland decreases. [4] In addition, pregnancy can modify a number of concomitant dermatoses and there are some pathological skin conditions that are virtually pregnancy specific.
The most common physiological changes are pigmentary alterations, stretch marks, vascular spiders and telogen effluvium. [5] In our study, 91.4% of cases had hyperpigmentation, the most common being linea nigra seen in 91.4% cases. Secondary areola developed in 78.4% cases. The other sites of increased localized pigmentation were seen over the abdomen, face, buttocks, scar pigmentation, breasts, axillae, neck in that order. Generalized darkening of skin was reported in 4 (0.66%) cases. These findings are comparable to other studies. [4],[6],[7]
Type b pigmentary demarcation lines are known to appear for the first time during pregnancy. [8] James et al . [9] in their study found 7 pregnant women who developed pigmentary demarcation line for the first time on the posteromedial aspect of lower limb. In our study, one woman developed pigmentary demarcation lines on the posterior aspect of lower limbs for the first time. Another woman reported it for the first time on the medial aspect of both forearms.
Melasma was seen in 15/607 (2.5%) of our cases. It has been reported to occur in 50-75% of pregnant women. [10] The onset in 12 (80%) cases was in the first trimester whereas Martin and Leal-Khouri [5] reported an onset of melasma mostly during the second trimester. Wong and Ellis [11] reported melasma in 50-70 % of pregnant women with an onset during the 2nd trimester. Of these, 72% cases were epidermal, 13% dermal and 5% were of the mixed type. In 30 % of cases, melasma tends to persist post partum. [10] Muzaffer et al [4] found melasma to be present in 65 (46.4%)of their cases. Raj et al [6] observed melasma in 10/1175 (8.5%) cases, which is closer to what is seen in our study. This difference may be due to the fact that pigmentary changes are more discernible in the fair skinned individuals.
Striae distensae (striae gravidarum) develop in up to 90% of women during the sixth and seventh month of pregnancy. [1] In our study, striae were seen in 484 (79.7%) cases of which 217 (44.8%) were primi gravidas and 267 (55.2%) were multi gravidas. Onset was most commonly seen during the second trimester. The most common site seen in primigravidas was lower abdomen and pink shiny striae were most common. Multi gravidas showed mostly white atrophic striae. Muzaffar et al . [4] found 77.1% (108/140) of their cases developed striae gravidarum. Raj et al . [6] also found striae distensae in 75% of pregnant women which is closer to that seen in our study. Chang et al . [12] found incidence of striae gravidarum in their survey to be 55%.
Striae are uncommon in Asian and African-American women and there seems to be a familial tendency. [1] Physical factors (stretching secondary to increase in the abdominal girth) play a role in the development of the striae. [11],[13] Lower birth weight and smaller babies as compared to Caucasian women may be related to 123/607 (20.3%) pregnant women not developing striae in our study. Poindevin et al . [14] in 1959, observed that women with lighter complexions had a greater tendency to develop striae compared with women of darker complexions.
Of the 607 women, 11 gave a history of increased hair loss and only 5 patients noticed lengthening and improvement in their scalp hair, whereas 591 (97.4%) gave history of no change in hair density. Muzaffar et al . [4] reported hair changes in 18 (12.8%) cases. Out of those 18 cases, diffuse thinning of scalp hair was noted in 7 (38.9%) cases. Nine (50%) patients noticed lengthening and improvement in their scalp hair. Frontoparietal recession and hypertrichosis was seen in one case each.
Increased appearance of Montgomery′s tubercles is well known during pregnancy in 30-50% of pregnant women. [5] In our study, Montgomery′s tubercles were seen in 220 (36.3%) cases. This was found to be consistent with other studies. [5]
Vascular changes result from distention, instability and proliferation of vessels and regress postpartum. Non pitting edema of legs, eyelids, face and hands is present in about 50% of women during the third trimester. [10] The edema decreases during the day and is thought to be due to secondary sodium and water retention in conjunction with increased capillary permeability. [5] Vascular changes seen in our study include nonpitting edema of feet in 59 (9.8%) cases and abdominal wall edema in 3 cases.
Vascular spiders (spider angiomas, nevi aranei) appear in 67% of white and 11% of black women between the 2nd and 5th month of pregnancy. Palmar erythema occurs in 66% of white and 33% of black women beginning in first trimester. [5] No cases of palmar erythema were seen in our study which may be related to less visibility in darker skin. In a recent study by Muzaffar et al ., [4] palmar erythema was seen in 12.1% of cases, varicosities of lower legs in 2.8%, vascular spiders in 1.4% and cutis marmorata in 0.7% cases. Varicosities are most common in anus and legs, appearing in 40% of pregnant women during the 3rd trimester. [10] Raj et al . [6] noted varicose veins in 6 out of 1,175 women. Esteve et al . [7] observed vascular changes in 50 women including vascular spiders in 32 out of the 60 women.
During pregnancy, the gingivae enlarge, darken and become red and swollen in up to 80% of women. Edema and hyperemia due to hormonal changes as well as local irritation and nutritional deficiencies may be responsible. [5] Gingivitis not attributable to bad oral hygiene was seen in 9 out of 607 pregnant women. In a study by Muzaffar et al . [4] 23/140 (16.4%) had gingival edema and redness. Raj et al . [6] had seen 3 cases of pyogenic granulomas in their study.
Specific dermatoses of pregnancy are almost always associated with pruritus and an eruption of variable severity. Holmes and Black [15] proposed a simplified clinical classification of the specific dermatoses of pregnancy. This classification basically subdivided the specific dermatoses of pregnancy into four groups: (i) pemphigoid (herpes) gestationis (PG); (ii) polymorphic eruption of pregnancy (PEP); (iii) prurigo of pregnancy; and (iv) pruritic folliculitis of pregnancy (PF). [15] The incidence of these specific disorders of pregnancy is 0.5 to 3.0%. [16] In our study of 607 pregnant women, 22 (3.6%) cases of specific dermatoses of pregnancy were seen. Of these the most common was PUPPP (also known as polymorphic eruption of pregnancy) with a total of 63.6% (14/22) cases followed by 5 (22.7%) cases of pruritus gravidarum. In an Indian study, Shivakumar and Madhavamurthy [17] found pruritus to be the commonest symptom (58.82%). Candidiasis (21.78%) was the commonest cause of white discharge per vagina, Condylomata acuminata (4.70%) was the commonest sexually transmitted disease. Of the disorders specific to pregnancy, 16 (9.41%) had prurigo of pregnancy, 6 (3.52%) had pruritus gravidarum and 4 (2.35%) had polymorphic eruption of pregnancy.
This study brings into focus various skin changes during pregnancy. Clinicians need to distinguish between physiological skin changes and specific dermatoses of pregnancy for better patient care.
| 1. |
Kroumpouzos G, Cohen LM. Dermatoses of pregnancy. J Am Acad Dermatol 2001;45:1-19.
[Google Scholar]
|
| 2. |
Lawley TJ, Yancey KB. Skin changes and diseases in pregnancy. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, et al , editors. Fitzpatrick's Dermatology in general medicine. 5th ed. McGraw-Hill: New York; 1999. p. 1963-9.
[Google Scholar]
|
| 3. |
Winton GB. Skin diseases aggravated by pregnancy. J Am Acad Dermatol 1989;20:1-13.
[Google Scholar]
|
| 4. |
Muzaffar F, Hussain I, Haroon TS. Physiologic skin changes during pregnancy: A study of 140 cases Int J Dermatol 1998;37:429-31.
[Google Scholar]
|
| 5. |
Martin AG, Leal-Khouri S. Physiological skin changes associated with pregnancy. Int J Dermatol 1992;31:375-8.
[Google Scholar]
|
| 6. |
Raj S, Khopkar U, Kapasi A, Wadhwa SL. Skin in pregnancy. Indian J Dermatol Venereol Leprol 1992;58:84-8.
[Google Scholar]
|
| 7. |
Esteve E, Saudeau L, Pierre F, Barruet K, Vaillant L, Lorette G. Physiological cutaneous signs in normal pregnancy: A study of 60 pregnant women. Ann Dermatol Venereol 1994;121:227-31.
[Google Scholar]
|
| 8. |
Ruiz-Villaverde R, Blasco Melguizo J, Naranjo-Sintes R. Pigmentary demarcation lines in a pregnant Caucasian woman. Int J Dermatol 2004;43:911-2.
[Google Scholar]
|
| 9. |
James WD, Carter JM, Rodman OG, Antonio S. Pigmentary demarcation lines: A population survey. J Am Acad Dermatol 1987;16:584-90.
[Google Scholar]
|
| 10. |
Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol 1982;6:977-98.
[Google Scholar]
|
| 11. |
Wong RC, Ellis CN. Physiologic changes in pregnancy. J Am Acad Dermatol 1984;10:929-40.
[Google Scholar]
|
| 12. |
Chang AL, Agredano YZ, Kimball AB. Risk factors associated with sriae gravidarum. J Am Acad Dermatol 2004;51:881-5.
[Google Scholar]
|
| 13. |
Shuster S. The cause of striae distensae. Acta Derm Venereol Suppl 1979;59:161-9.
[Google Scholar]
|
| 14. |
Poindevin LO. Striae gravidarum: Their relation to adrena cortical hyperfunction. Lancet 1959;2:436-9.
[Google Scholar]
|
| 15. |
Holmes RC, Black MM. The specific dermatoses of pregnancy: A reappraisal with special emphasis on a proposed simplified clinical classification. Clin Exp Dermatol 1982;7:65-73.
[Google Scholar]
|
| 16. |
Roger D, Vaillant L, Fignon A, Pierre F, Bacq Y, Brechot JF, et al . Specific pruritic dermatoses of pregnancy: A prospective study of 3192 women. Arch Dermatol 1994;130:734-9.
[Google Scholar]
|
| 17. |
Shivakumar V, Madhavamurthy P. Skin in pregnancy. Indian J Dermatol Venereol Leprol 1999;65:23-5.
[Google Scholar]
|
Fulltext Views
8,257
PDF downloads
5,032





