Translate this page into:
A clinico-epidemiological study of adult acne: Is it different from adolescent acne?
Correspondence Address:
Niti Khunger
Department of Dermatology and STD, VM Medical College and Safdarjang Hospital, New Delhi
India
| How to cite this article: Khunger N, Kumar C. A clinico-epidemiological study of adult acne: Is it different from adolescent acne?. Indian J Dermatol Venereol Leprol 2012;78:335-341 |
Abstract
Background: Although acne is usually recognized as an adolescent skin disorder, the prevalence of adults with acne is increasing. There is surprisingly a paucity of data on the prevalence and clinical features of postadolescent acne in the adult Indian population. Aims: The clinical and epidemiological data of adult acne were evaluated with a view to establishing possible contributing etiological factors and observing whether clinical features differ from adolescent acne. Methods: Patients over the age of 25 years presenting with acne in a tertiary care hospital were included in the study. A detailed history and examination was carried out, with a stress on aggravating factors. Hormonal imbalances were investigated in females with alopecia, obesity, hirsutism and menstrual irregularity. Severity of acne and complications like scarring and psychological stress were included. Results: Out of 280 patients included in the study 82.1% were women and 17.9% were men. The mean age of the patients was 30.5 years. Persistent acne was observed in 73.2%, while it was late onset in 26.8%. Majority of the patients had inflammatory papular acne (55%), whereas comedonal acne was the least common (6%). Most common predominant site of involvement was cheek (81%), followed by chin (67%), and mandibular area (58.3%). Family history of acne was present in 38.6%. Premenstrual flare was seen in 11.7% of female patients, obesity in 6.4%, hirsutism in 5.7% and alopecia in 1.8%, but raised laboratory markers of hyperandrogenism were observed in only 3.08%. Scarring was observed in a majority of patients (76.4%) and psychological stress in 52.8% patients. Conclusion: Adult acne is predominant in women, and as compared to adolescent acne is more inflammatory, with involvement of the cheeks and lower half of the face, while comedones are rare. Facial scarring occurs in a majority and stress is common, which emphasizes that adult acne should not be neglected.Introduction
Acne is one of the most common skin disorders worldwide and occurs primarily at puberty with a prevalence of almost 95%. [1] Although acne is principally a disorder of adolescence, the prevalence of adult patients with acne is increasing. [2] Adult acne has been traditionally defined as presence of acne beyond the age of 25 years. [2] There are two types of adult acne; persistent acne and late-onset acne. Adolescent acne persisting beyond the age of 25 years is called persistent adult acne and acne developing for the first time after the age of 25 years is called as late-onset adult acne. Both the types are more common in women. In persistent acne, patients have lesions on most days and may experience a premenstrual flare. Late onset acne can be further subdivided into chin acne, which occurs around the chin and perioral area, is inflammatory and flares premenstrually and sporadic acne which occurs suddenly in adult life with no distinguishing features. [3] It has been reported that patients with adult acne have a 40% prevalence of psychiatric comorbidity. [4] There is surprisingly, relatively few data on the prevalence and studies of acne in the adult population and to our knowledge none in the Indian population. The objectives of this study were to observe the prevalence, clinical features, contributing etiological and aggravating factors of acne in adults, with a view to establishing possible etiological factors and highlight possible differences from adolescent acne.
Methods
The study was conducted over a period of 18 months from November 2008 to April 2010. Ethical clearance was not obtained as it was an observational study. Patients over the age of 25 years presenting with acne vulgaris in the outpatient department of dermatology in a tertiary care hospital in north India were included in the study. A detailed history and examination was carried out for each patient, including a medical and family history. Information about the extent and site of involvement, aggravating factors including drug intake, sun exposure, seasonal variation, application of cosmetics, stress, premenstrual flare and influence of pregnancy on acne was obtained. Gynecological and obstetrical history of female patients was noted. Clinical assessment of each patient included type of acne lesions, distribution, severity and grading of acne. Acne vulgaris was graded using a simple system taking into account the predominant lesions present; thus classifying acne into four grades. [5]
Grade 1: Comedones, occasional papules.
Grade 2: Papules, comedones, few pustules.
Grade 3: Predominant pustules, nodules, abscesses.
Grade 4: Mainly cysts, abscesses, widespread scarring.
Acne scars were graded into mild, moderate and severe according to the qualitative grading system. [6]
Rosacea was excluded on the basis of clinical examination. Associated findings such as obesity, hirsutism and alopecia indicating hormonal imbalance were also noted and investigations for polycystic ovarian disease and insulin resistance were done when indicated. Hormonal evaluation in terms of serum level of total testosterone, serum dehydroepiandrosterone (DHEAS), luteinising hormone (LH), follicular stimulating hormone (FSH) and prolactin (PRL) were done. Stress was evaluated through a questionnaire answered by the patient. A subjective assessment of its association with aggravation of the acne was made by the patient. Results were derived by simple statistical means of percentage and proportions.
Results
The study included 280 patients, 230 (82.1%) women and 50 (17.9%) men, with a M:F=1:4.6. The prevalence of adult acne presenting for treatment in our study was found to be 0.38% (280 patients out of 72710 patients attending the OPD during the study duration). The mean age of the patients was 30.5 years with a range of 26-50 years. The proportion of population of different age groups is detailed in [Table - 1].
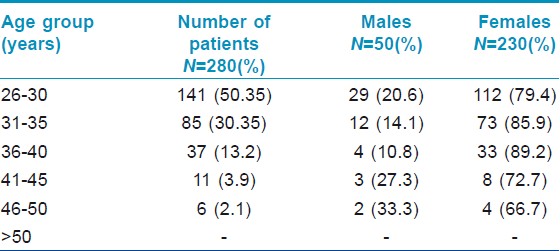
The duration of acne varied from minimum of 2 weeks to as long as 30 years. Proportion of patients having persistent acne was 73.2% (205 patients comprising of 160 females and 45 males) while those having late onset acne was 26.8% (75 patients comprising of 73 females and 2 males). There was no difference in clinical features between persistent acne and late onset acne. Majority of the patients were having acne grade II (55%) followed by grade III (28%), grade IV (12%) and grade I (6%) [Figure - 1]. Facial involvement was seen in all, except six patients in whom only truncal involvement was present. On the face, cheek was the most common site involved (81%) followed by chin (67%), mandibular area (58.3%), forehead (51.7%) and nose (18.3%) [Figure - 2].
 |
| Figure 1: Severity according to grade of acne |
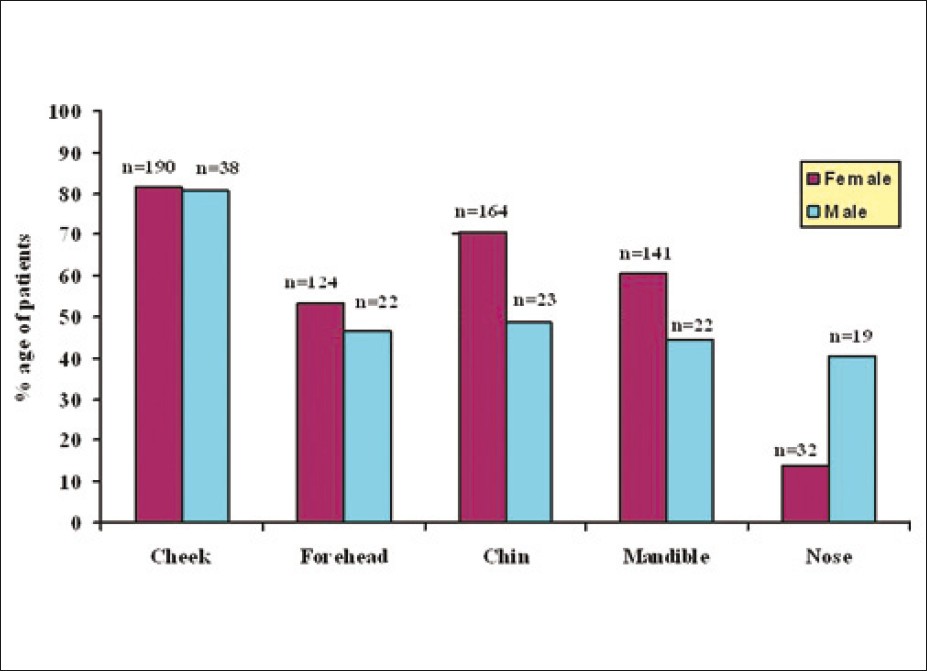 |
| Figure 2: Gender - wise distribution of acne over face |
Onset of acne due to drug use was found in only three cases (Two due to antitubercular therapy and one due to antipsychotic medications). Topical steroid use was found to be an important factor responsible for aggravation of acne in our study population (11.8%). Family history of acne with first degree relative involved was found in 108 (38.6%) patients. Seasonal aggravation in acne was noted, summer being the most common (36.4%) followed by rainy season (5.0%) and winter season (1.4%) season. Exposure to sunlight was also responsible for aggravation in 93 (33.2%) patients. Regular use of cosmetics was present in 178 (63.5%) patients with aggravation of acne due to cosmetics in 40 (14.3%) patients. Aggravation due to stress was seen in 72 (25.7%) of our patients. Premenstrual flare was seen in 27 (11.7%) of female patients. Associated conditions such as obesity were observed in 6.4%, hirsutism in 5.7% and alopecia in 1.8%. Laboratory evidence of hyperandrogenism in the form of raised testosterone levels was seen in only 7 out of 230 female patients (3.04%). Serum DHEAS and PRL were not deranged in any patient. Serum LH was found to be raised in 3 patients. Serum FSH was decreased in one patient, the ratio of LH:FSH was >2:1 in 2 patients, favouring polycystic ovarian syndrome. Ultrasonography report of two patients favoured a diagnosis of polycystic ovarian disease (PCOD). Only two patients had raised level of insulin, indicating insulin resistance. Pregnancy was associated with worsening of acne in 11 (5.6%) female patients while improvement was observed in 14 (7.1%) out of 197 females who underwent a pregnancy, while the rest (87.3%) reported no significant change in acne during pregnancy.
Complications of acne studied included facial scarring and psychosocial stress. Acne scarring was observed in a majority of patients (76.4%). It was mild in 149 (69.6%) patients followed by moderate in 60 (28.1%) and severe in 5 (2.3%) patients. Majority of the scars were persistent pigmented macular scars (44.3%) followed by icepick (28.6%), rolling atrophic (27.1%) and keloidal (1%) types [Figure - 3]. Psychological stress due to acne was reported in 148 (52.8%) patients.
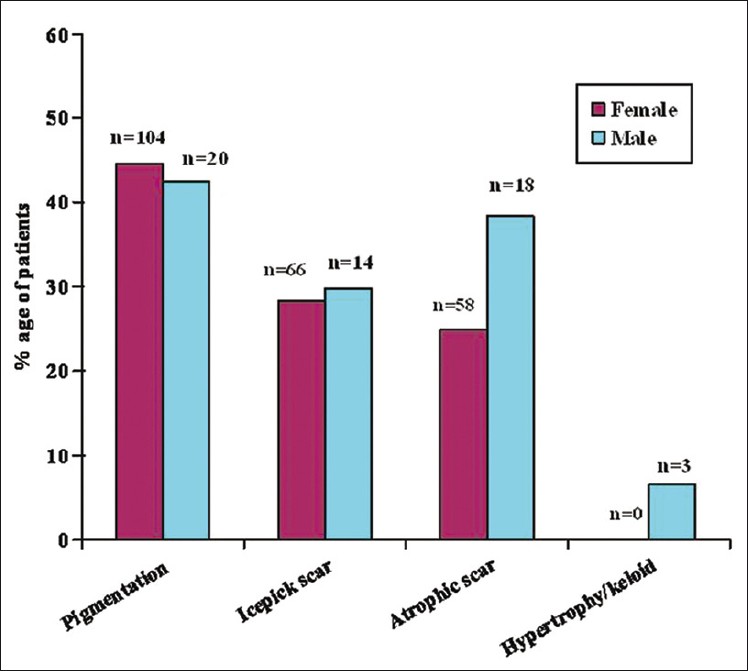 |
| Figure 3: Scarring in acne |
Discussion
Acne is traditionally considered as a disease of adolescent age and there are very few reports on postadolescent or adult acne. In a hospital-based Indian study, 9.4% patients (29 out of 309) were more than 25 years. [7] Although acne usually declines after the age of 40 years, in our study, it was still present in 2.1% of patients beyond 45 years. In comparison, Goulden et al., [2] reported acne in 12.5% beyond 45 years. Adult acne is more common in women. [2],[3],[8],[9] Similarly, in our study women were predominantly affected (82.1%) as compared to men (17.9%). This may represent an increased awareness in women seeking treatment as compared to men. However, a community-based survey of more than 700 adults older than 25 years also reported clinical facial acne in 12% of women and 3% of men. [2] Hormonal factors, increased use of cosmetics and exposure to hot and humid conditions while cooking may play a role in increased prevalence of adult acne in women.
Adult acne can either persist from adolescence or be late onset beginning in adulthood. In our study acne was persistent from adolescence in a majority of patients (73.2%) which is in concordance with the study of Goulden et al., [2] where 82% of the population had persistent acne. Thus in a majority, acne develops in adolescence and persists into adulthood. Gender-wise late onset adult acne is predominant in women (97.3%) as compared to men (2.7%). Morphologically, the classical presentation of adult acne has been described as inflammatory papulopustular lesions in the mandibular and chin area. [2],[9],[10] Capitanio et al., [11] described another variant called comedonal postadolescent acne, in which inflammatory lesions were sparse but large prominent cyst-like comedones were homogeneously distributed on the whole face with a relative absence on the lower third of the face and the jawline. This variant was common in smokers. Smoking as an etiological factor was not studied in our patients. Our study revealed that clinically, adult acne in Indian patients usually presents as inflammatory papules and pustules (83%) and is mild to moderate in severity (grade II in 55% of patients and grade III in 28% of patients) [Figure - 4]. Comedonal acne is rare as compared to adolescent acne. However, cystic acne is not uncommon and was still present in 12% of adult patients [Figure - 5]. Similar results were observed by Goulden et al., [2] where postadolescent acne was predominantly inflammatory.
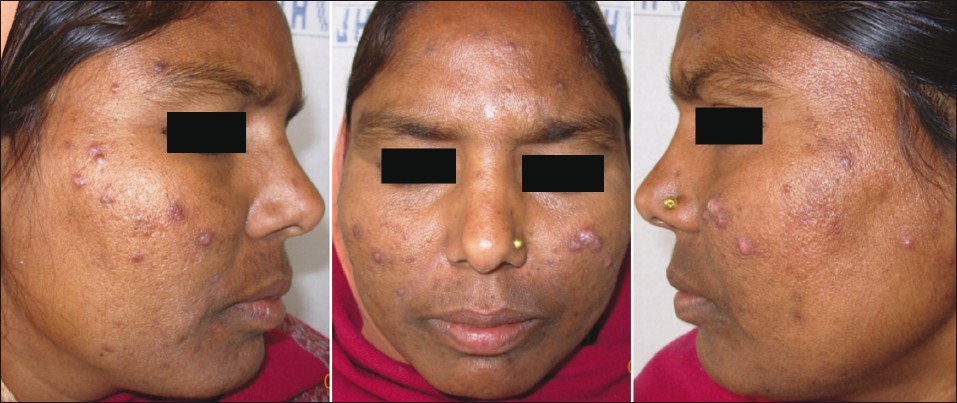 |
| Figure 4: Inflammatory papulo-pustular acne on the cheeks in a 34-year-old woman |
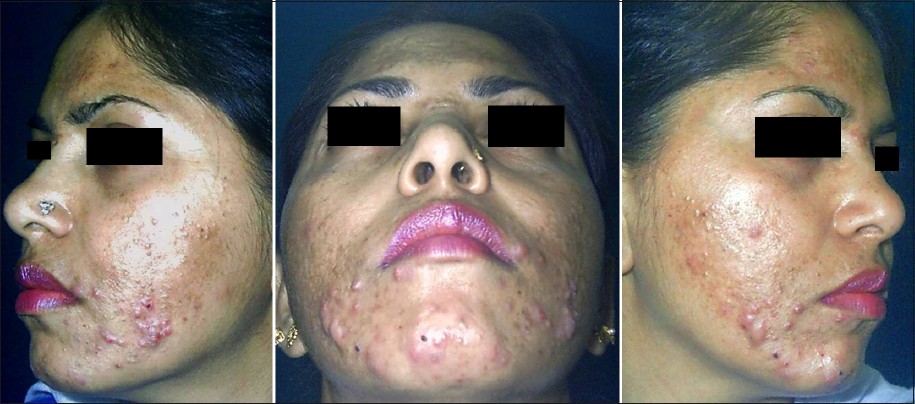 |
| Figure 5: Nodulocystic acne in the chin and mandibular area in a 28 - year - old woman |
Postadolescent acne is predominantly facial and in our study only six patients (2.1%) had only truncal involvement without facial involvement. This may also be due to the fact that pure truncal involvement is not cosmetically distressing, hence fewer patients present for treatment. In our study a surprising finding was that involvement of the cheek was the most common site on face in comparison to involvement of mandibular area. This is in contrast to studies from western countries that have reported involvement of lower face and mandibular area more commonly in adult acne cases. [2],[10],[11]
The causes of postadolescent acne remain to be fully elucidated and hormones, colonization by resistant bacteria, the use of cosmetics, drugs and chronic stress have been put forward as possible etiological factors. Since adult acne is more common in women, there has always been an emphasis on underlying hormonal imbalance. The role of circulating androgen levels in adult acne still remains controversial. In our study, features suggestive of hyperandrogenism such as hirsutism was observed in 6.95% and alopecia in 2.17%. However, although clinical features suggestive of hyperandrogenism such as premenstrual flare (11.7%), hirsutism (5.7%) and alopecia (1.8%) were present, only 7 (3.04%) women had raised laboratory markers of hyperandrogenism. In the study by Goulden et al., [2] 37% of women had at least one feature of possible hyperandrogenism as characterized by hirsutism (24.2%), alopecia (7.2%) or menstrual disturbance (17.7%). In our study, premenstrual flare was observed in 11.7%, whereas Goulden et al., [2] observed premenstrual increase in 84.8%. Polycystic ovaries have been reported in 52-82% of patients with adult acne, but hormonal profiles were not in keeping with the classical polycystic ovary syndrome. [12] Another study showed that 11.8% of patients with post adolescent acne had reduced activity of the enzyme 21-hydroxylase, suggesting late-onset congenital adrenal hyperplasia. [13] In another study of 13 patients with late onset adult acne, underlying abnormalities in levels of androgens were not observed, but onset was related to stress. [14] These patients failed to respond to antibiotics and to isotretinoin, but five of the patients had clearance of acne after fluoxetine was prescribed for depression. Kligman [15] proposed that perhaps the major etiological factor in adult acne was increased levels of stress, leading to increase in adrenal androgens. Another study reported finding significantly higher levels of adrenal testosterone and DHT in adult women with acne compared with controls. [16] In a contrasting study, adrenal androgens in 9 women with postadolescent idiopathic acne were not significantly raised as compared to age and sex-matched normal controls. [17] Studies concluded that adrenal androgen secretion is only mildly elevated in patients with post adolescent acne but patients without acne may have similar hormone levels. [17],[18] These contrasting findings suggest that end-organ hypersensitivity and not androgen levels may be the central factor in development of adult acne in women. [19] Increased sensitivity of the sebaceous gland to androgens or increased local metabolism of androgen hormones in the skin to potent androgen metabolites may offer alternative mechanisms for the pathogenesis of this disorder. [17] More so, studies are there to support the concept that target tissue androgens might play a major role in pathogenesis of female acne. [20] It is possible that a certain number of follicles are acne-prone and these follicles have different levels of susceptibility to the circulating hormones.
It has been suggested that chronic stress might be a possible cause of increased androgen secretion in some of the women, resulting in the pathogenesis of acne in such patients. [15] Several such reports have been published regarding the association of increased level of cortisol and emotional stress. [21] In our study, 25.7% patients reported on stress as an aggravating factor, in contrast to Goulden et al., [2] who reported that in 71% of their patients acne flared with stress.
In our study, pregnancy was associated with worsening of acne in 5.6%, while improvement was seen in 7.1%, and a majority observed no change. This is in contrast to the study by Goulden et al., [2] where pregnancy resulted in a flare in 18%, improvement in 43%, and had no effect in 39%.
Steroids have been implicated in causation of acne by inducing hypercornification of the upper portion of pilosebaceous unit. There was no significant relation with drug usage in our patients, except the use of topical steroids which caused aggravation in all the patients applying it (11.8%). In three of our patients it was evident that the acne was precipitated due to drug use (2 due to anti-tubercular therapy and 1 due to anti psychotic medications). Similar results were reported by Goulden et al., [2] where prednisolone, lithium, anabolic steroids and phenytoin were causative factors in five patients.
Cosmetic induced acne has been originally discussed as an important cause of mild to moderate acne in female subpopulation. [22] In our study only 40 patients (22%) out of 176 using some form of cosmetics, reported aggravation due to cosmetic use. Similarly, other studies do not reveal cosmetic as an etiological factor in adult acne.
It has been traditionally believed that acne improves in summer and aggravates in winter. However, in our study, sun exposure and summer season were found as aggravating factors in 32% and 36.7% of our cases respectively. Similarly, an Indian study observed that in a majority (80.62%) of patients a summer aggravation of acne was seen. [23] It is possible that UV radiation, which may cause inflammation and generate squalene peroxides which are highly comedogenic, may play a role in the persistence of acne in tropical countries, in addition to sweating and increased humidity. [24],[25] No differences in bacterial flora have been observed between adolescent and adults with acne, hence antibiotic resistant bacteria and high antibody levels to P. acnes seem unlikely to be involved in the pathogenesis of postadolescent acne. [26]
It has been suggested that acne might be familial according to the results of a large study of identical twins showing 97.9% of affection. [27] In our study population, 38.8% patients had at least one first degree relative affected with clinical acne, while Goulden et al., [2] observed a higher incidence of 50%. Hence it seems to be true that genetic factors might play role in acne development and persistence.
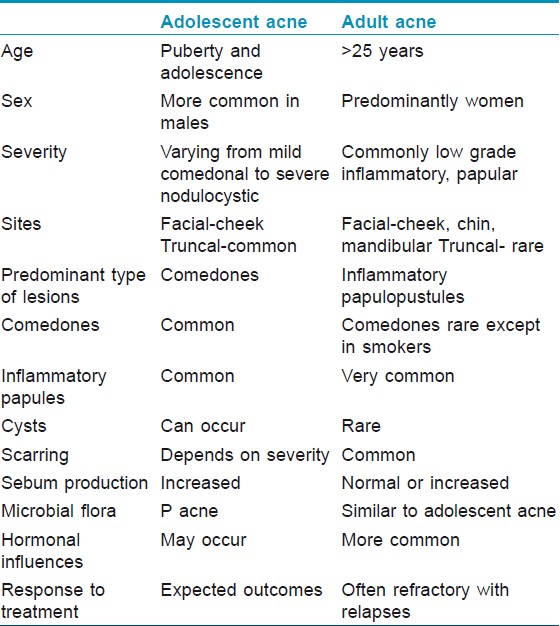
Scarring in adult acne is more common as compared to adolescent acne. This is probably because acne in adults is predominantly inflammatory, may be resistant to treatment and treatment is often delayed, which leads to greater scarring. Scarring was very common in our patients (76.4%), with predominantly persistent pigmented lesions followed by icepick and rolling atrophic scars. One Indian study for acne of all groups documented scarring and hyperpigmentation in 39.5% and 24.6% of patients respectively. [7] Goulden et al., [2] observed scarring in 77.2% men and 58.5% women and icepick scars were the commonest type. Thus there are no predominant etiopathogenetic factors in adult acne. The differences between adolescent acne and adult acne are highlighted in [Table - 2].
Patients with adult acne are now being recognized as an increasingly important population requiring treatment. Though inflammatory lesions are more refractory to treatment, in most cases, prescribed therapies are very similar to those used for adolescent acne. Hormonal therapy should not be the first line for acne treatment, but is appropriate for women with raised androgen levels or signs of hyperandrogenism, refractory acne and those who also require contraception. [19] The chief limitation of our study is that response to treatment was not included in the observations.
Conclusions
Acne as a disease is lasts longer, persists into adulthood and requires treatment well into the forties. Patient with post adolescent acne remains a challenge to treat. There are hardly any well-documented studies that define why acne persists or begins in adulthood, why adult women continue to be affected with acne significantly more than adult men and what is the role of etiological and aggravating factors in the presence of acne well beyond adolescence. To the best of our knowledge this is the first Indian study on adult acne. Unlike teenage acne, where males tend to be affected more commonly and show the most severe forms of the disease, post-adolescent acne mainly affects females and is more inflammatory in nature. It is predominantly facial and unlike previous studies, in Indian patients, cheeks are still the commonest predominant site of involvement. Facial scarring occurs in a majority of patients and emphasizes the fact that adult acne should be adequately treated.
| 1. |
Burton JL, Cunliffe WJ, Stafford I, Shuster S. The prevalence of acne vulgaris in adolescence. Br J Dermatol 1971;85:119-26.
[Google Scholar]
|
| 2. |
Goulden V, Clark S, Cunliffe W. Post-adolescent acne: A review of clinical features. Br J Dermatol 1997;136:66-70.
[Google Scholar]
|
| 3. |
Williams C, Layton AM. Persistent acne in women. Implications for the patient and for therapy. Am J Clin Dermatol 2006;7:281-90.
[Google Scholar]
|
| 4. |
Henkel V, Moehrenschlager M, Hegerl U, Moeller HJ, Ring J, Worret WI. Screening for depression in adult acne vulgaris patients: Tools for the dermatologist. J Cosmet Dermatol 2002;1:202-7.
[Google Scholar]
|
| 5. |
Tutakne MA, Chari KV. Acne, rosacea and perioral dermatitis. In: Valia RG, Valia AR, editors. IADVL Textbook and Atlas of Dermatology. 2 nd ed. Mumbai: Bhalani Publishing House; 2003. p. 689-710.
[Google Scholar]
|
| 6. |
Goodman G, Baron JA. Post acne scarring a qualitative global scarring grading system. Dermatol Surg 2006;32:1458-66.
[Google Scholar]
|
| 7. |
Adityan B, Thappa DM. Profile of acne vulgaris-A hospital-based study from South India. Indian J Dermatol Venereol Leprol 2009;75:272-8.
[Google Scholar]
|
| 8. |
Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol 1999;41:577-80.
[Google Scholar]
|
| 9. |
Shaw JC, White LE. Persistent acne in adult women. Arch Dermatol 2001;137:1252-3.
[Google Scholar]
|
| 10. |
Poli F, Dreno B, Verschoore M. An epidemiological study of acne in female adults: Results of a survey conducted in France. J Eur Acad Dermatol Venereol 2001;15:541-5.
[Google Scholar]
|
| 11. |
Capitano B, Sinagra JL, Bordignon V, Fei PC, Picardo M, Zouboulis CC. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol 2010;63:782-8.
[Google Scholar]
|
| 12. |
Betti R, Bencini PL, Lodi A, Urbani CE, Chiarelli G, Crosti C. Incidcnce of polycystic ovaries in patients with late-onset or persistent acne: Hormonal reports. Dermatotogica 1990;181:109-11.
[Google Scholar]
|
| 13. |
Oslere LS, Richardson T, Caine S. The prevalence of late onset congenital adrenal hyperplasia in females with post adolescent acne. Br J Dermatol 1993;129 Suppl 42:25.
[Google Scholar]
|
| 14. |
Harrington CI. Post-adolescent acne and marital break up. Br J Dermatol 1997;137:478-9.
[Google Scholar]
|
| 15. |
Kligman AM. Post-adolescent acne in women. Cutis 1991;48:75-7.
[Google Scholar]
|
| 16. |
Aizawa H, Niimura M. Adrenal androgen abnormalities in women with late onset and persistent acne. Arch Dermatol Res 1993;284:451-5.
[Google Scholar]
|
| 17. |
Chrousos GP, Peck GL, Gross EG, Cutler GB Jr, Loriaux DL. Adrenal function in women with idiopathic acne. J Invest Dermatol 1982;78:468-71.
[Google Scholar]
|
| 18. |
Thiboutot D, Gilliland K, Light J, Lookingbill D. Androgen metabolism in sebaceous glands from subjects with and without acne. Arch Dermatol 1999;135:1041-5.
[Google Scholar]
|
| 19. |
Knaggs HE, Wood EJ, Rizer RL, Mills OH. Post-adolescent acne. Int J Cosmet Sci 2004;26:129-38.
[Google Scholar]
|
| 20. |
Carmina E, Lobo RA. Evidence for increased androsterone metabolism in some normoandrogenic women with acne. J Clin Endocrinol Metab 1993;76:1111-4.
[Google Scholar]
|
| 21. |
Laue L, Peck GL, Loriaux DL, Gallucci W, Chrousos GP. Adrenal androgen secretion in post adolescent acne: Increased adrenocortical function without hypersensitivity to adrenocorticotropin. J Clin Endocrinol Metab 1991;73:380-4.
[Google Scholar]
|
| 22. |
Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol 1972;106:843-50.
[Google Scholar]
|
| 23. |
Sardana K, Sharma RC, Sarkar R. Seasonal variation in acne vulgaris-Myth or reality. J Dermatol 2002;29:484-8.
[Google Scholar]
|
| 24. |
Mills OH, Porte M, Kligman AM. Enhancement of comedogenic substances by ultraviolet radiation. Br J Dermatol 1978;98:145-50.
[Google Scholar]
|
| 25. |
Motoyoshi K. Enhanced comedo formation in rabbit ear skin by squalene and oleic acid peroxides. Br J Dermatol 1983;109:191-8.
[Google Scholar]
|
| 26. |
Till AE, Goulden V, Cunliffe WJ, Holland KT. The cutaneous microflora of adolescent, persistent and lateonset acne patients does not differ. Br J Dermatol 2000;142:885-92.
[Google Scholar]
|
| 27. |
Goulden V, Mcgeown CH, Cunliffe WJ. The familial risk of adult acne: A comparison between first-degree relatives of affected and unaffected individuals. Br J Dermatol 1999;141:297-300.
[Google Scholar]
|
Fulltext Views
12,621
PDF downloads
2,786





