Translate this page into:
A cross-sectional analysis of the effects of various centrifugation speeds and inclusion of the buffy coat in platelet-rich plasma preparation
Corresponding author: Dr. Varadraj Vasant Pai, Department of Dermatology, Goa Medical College, Bambolim, Goa, India. docpai@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Muthuprabakaran K, Pai VV, Ahmad S, Shukla P. A cross-sectional analysis of the effects of various centrifugation speeds and inclusion of the buffy coat in platelet-rich plasma preparation. Indian J Dermatol Venereol Leprol 2021;87:792-9.
Abstract
Introduction:
Platelet-rich plasma is an autologous blood preparation which is used in various medical specialties because of its regenerative properties. There is a wide variation in platelet-rich plasma preparation protocols and attaining the ideal platelet yield (>1 million platelets/μL) in a clinic setting can be challenging. We aimed at analyzing the centrifuge spin rates at which to attain an ideal platelet-rich plasma yield and also to study the effect of inclusion of the buffy coat after the first spin on the final platelet concentration in platelet-rich plasma.
Methods:
Seventy-five whole blood samples were obtained and divided into two groups – (1) leukocyte-rich platelet-rich plasma group and (2) leukocyte-poor platelet-rich plasma group. Samples in both groups were centrifuged using the dual spin method, at one of three centrifugation speed combinations (initial “soft” spin and second “hard” spin speeds, respectively): (1) 100 g/400 g, (2) 350 g/1350 g and (3) 900 g/1800 g. Platelet, red blood cell (RBC) and white blood cell (WBC) counts in both groups were compared.
Results:
The 100 g/400 g spin gave a high platelet yield (increase of 395.4 ± 111.1%) in the leukocyte-poor-platelet-rich plasma group, while in the leukocyte-rich platelet-rich plasma group both 100 g/400 g and 350 g/1350 g spins resulted in significantly higher yields with an increase of 691.5 ± 316.3% and 738.6 ± 193.3%, respectively.
Limitations:
The study was limited by a smaller sample size in the pure platelet-rich plasma (leukocyte-poor platelet-rich plasma) group.
Conclusion:
Ideal platelet yields can be achieved with both the 100 g/400 g as well as the 350 g/1350 g spins using the buffy coat inclusion method while the 100 g/400 g spin for “pure” platelet-rich plasma accomplishes a near-ideal platelet count with significantly reduced contamination with other cells.
Keywords
Buffy coat
centrifugation speed
platelet count
platelet-rich plasma
Plain Language Summary
Platelets are blood cells important in blood clotting. Platelet-rich plasma is a concentrated extract of platelets obtained from blood and is used to treat various conditions, including skin disorders. Platelet-rich plasma is usually obtained from a blood sample using two centrifugation steps in a centrifuge machine. This study was to determine the centrifugation speeds which extract the highest concentration of platelets in platelet-rich plasma. The authors studied three centrifugation speed combinations and found that 100 g/400 g and 350 g/1350 g speeds (numbers are indicative of the speeds at the first and second centrifugation steps, g being a unit of centrifugal force generated in the centrifuge machine) give the most desirable results (around a 4- to 7-fold increase on average in the concentration of platelets, as compared to their initial concentration in the blood samples).
Introduction
Platelet-rich plasma is an autologous preparation of platelets in concentrated plasma (with usually >1,000,000 platelets/μL or 2–7 times the concentration in whole blood).1 Initial uses of platelet-rich plasma included maintaining hemostasis during surgery and for platelet transfusions in thrombocytopenic disorders.2 It has now attracted attention in several medical specialties such as orthopedics, maxillofacial surgery, regenerative medicine and dermatology, because of its ability to promote tissue regeneration and wound repair.3,4 Since platelet-rich plasma is autologous in nature and its extraction is minimally invasive, affordable and without major side effects, its therapeutic profile has expanded to include many dermatologic indications such as chronic ulcers, scar treatment, alopecia and skin rejuvenation.
Platelet-rich plasma can be classified into four groups according to the concentration of cellular components in it: leukocyte-poor platelet-rich plasma or pure platelet-rich plasma, leukocyte-rich platelet-rich plasma, pure platelet-rich fibrin and leukocyte and platelet-rich fibrin.5 The clinical indications for leukocyte-rich platelet-rich plasma and leukocyte-poor platelet-rich plasma in dermatology vary. Leukocyte-rich platelet-rich plasma is found effective in chronic ulcers of varied etiologies, acne scars and scar rejuvenation, while leukocyte-poor platelet-rich plasma shows good results in vitiligo and facial rejuvenation.6
Various factors that affect the utility of platelet-rich plasma in different conditions include the whole blood platelet count, injected dose of platelets, platelet-rich plasma leukocyte count, platelet-rich plasma preparation techniques, platelet activation methods, concentrations of platelet-released factors and use of automated or manual methods.2 Although platelet-rich plasma is being used since a long time, there is a wide variation in preparation protocols and attaining a count of >one million platelets/μL without using expensive platelet-rich plasma kits in a clinic or even a tertiary care setting still remains a hurdle. Our aims were to analyze the centrifuge spin rates at which an ideal platelet-rich plasma yield can be attained and to study the effect of inclusion of the buffy coat after the first spin (leukocyte-rich platelet-rich plasma) on the final platelet concentration in platelet-rich plasma.
Methods
A cross-sectional observational study was conducted at a tertiary care center in Goa over a period of three months from December 2019 to February 2020. The study was cleared by the hospital ethical committee. A total of 75 samples from 32 apparently healthy individuals/patients above 18 years of age, of either gender, attending the inpatient and outpatient clinic over that period, who were receiving platelet-rich plasma injections as a treatment for androgenic alopecia, alopecia areata, or chronic ulcers were randomly collected for the study. Among participants who were analyzed more than one time, care was taken to allot different spin rate during every monthly visit. Informed consent was taken from the participants. Those who refused consent or patients on antiplatelet agents were excluded from the study.
Procedure
Twenty milliliters of whole blood were obtained by venepuncture, out of which 10 ml was transferred into two ethylenediaminetetraacetic acid tubes (BD Vacutainer ethylenediaminetetraacetic acid 6 ml 13 × 100 mm). One milliliter was sent for complete blood count and the rest (9 ml) used for platelet-rich plasma preparation. All the tests were done from the same lab. The remaining 10 ml was used for treatment purpose. The collected sample was centrifuged by two spins in a non-refrigerated centrifuge machine (Remi-8c) – a “soft” spin (at lower revolutions per minute [rpm]) initially, followed by a “hard” spin (at higher rpm). Various centrifugation speeds were considered based on the previous studies on determining optimum centrifugation speed in platelet-rich plasma preparation.7-12
The spins used in our study are given in Table 1. Following the first spin, three layers (red blood cells (RBC), buffy coat and plasma) were formed from whole blood. For the second spin, the sample was separated and transferred into sterile plain tubes (6 ml 13 × 100 mm) by either one of these two methods [Figure 1]:
| Spin variations | Soft spin | Hard spin | ||
|---|---|---|---|---|
| RCF (g)/rpm | Duration (min) | RCF (g)/rpm | Duration | |
| Variation 1 (25 samples) | 100 (900) | 10 | 400 (1800) | 10 |
| Variation 2 (25 samples) | 350 (1600) | 10 | 1350 (3200) | 10 |
| Variation 3 (25 samples) | 800 (2500) | 10 | 1600 (3500) | 10 |
Rpm values of corresponding RCF are given within brackets. RCF: Relative centrifugal force, rpm: Revolutions per minute
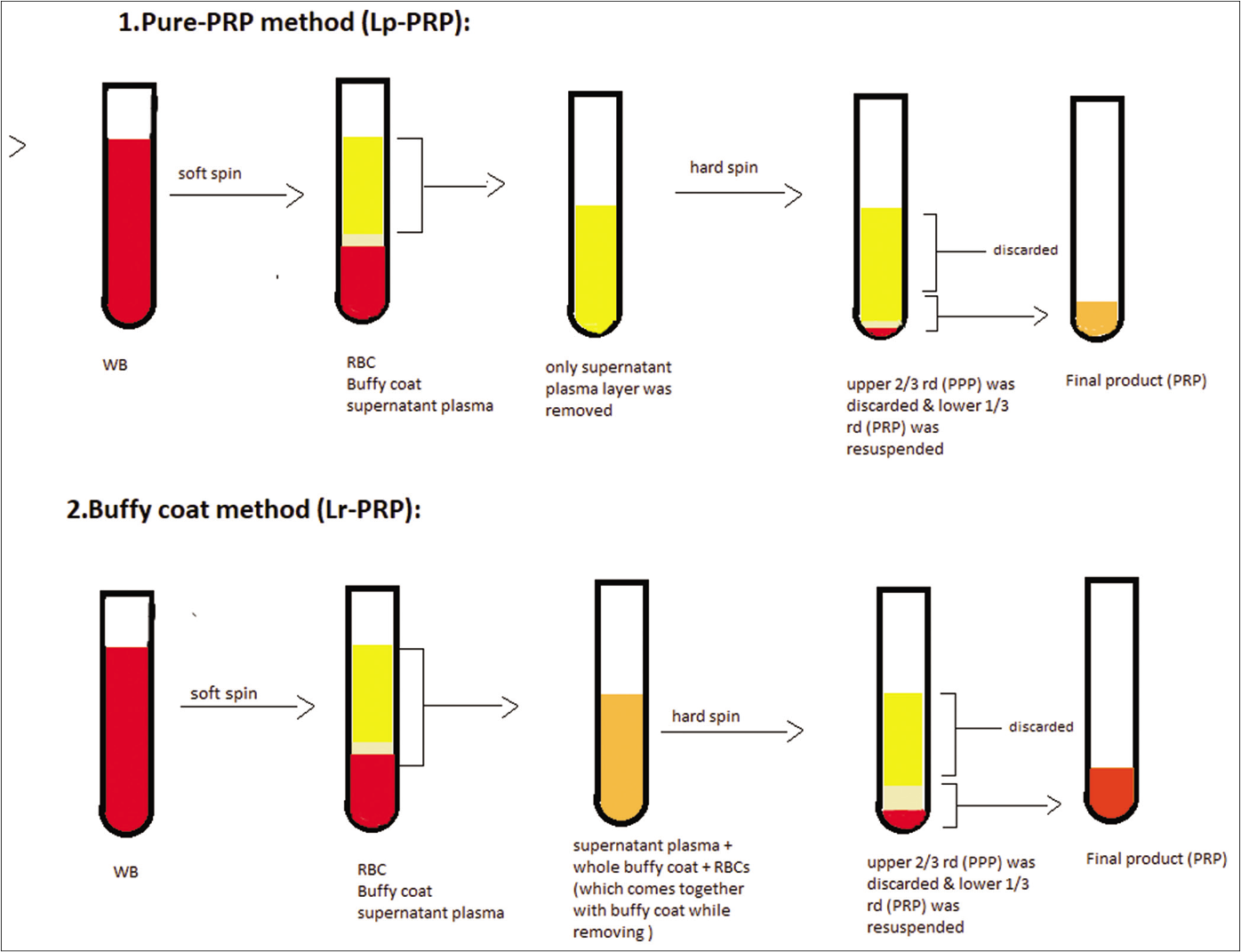
- Method of preparation of leukocyte-poor platelet-rich plasma and leukocyte-rich platelet-rich plasma
Pure platelet-rich plasma method (leukocyte-poor platelet-rich plasma): only the supernatant plasma layer was separated from the soft spin tube without disturbing the buffy coat or RBC layers, using a 2ml disposable syringe with 24G needle and subjected to a second spin. The duration of spin was ten minutes.13,19
Buffy coat method (leukocyte-rich platelet-rich plasma): in this method, the supernatant plasma was collected along with the whole buffy coat layer using a 2 ml disposable syringe with 24G needle and subjected to second spin. The duration of spin was ten minutes.
Table 2 shows the distribution of samples among the above two methods.
| Spin variations | Pure-PRP (Lp-PRP) (method 1) | Buffy coat (Lr-PRP) (method 2) | Total |
|---|---|---|---|
| Variation 1 (100 g/400 g) | 5 samples | 20 samples | 25 |
| Variation 2 (350 g/1350 g) | 5 samples | 20 samples | 25 |
| Variation 3 (800 g/1600 g) | 5 samples | 20 samples | 25 |
| Total | 15 samples | 60 samples | 75 |
PRP: Platelet-rich plasma, Lr-PRP: Leukocyte rich PRP, Lr-PRP: Leukocyte poor PRP
After the second spin, the upper two-third of the sample (platelet-poor plasma) was removed and the lower one-third was homogenized, by gentle shaking, to obtain platelet-rich plasma, which was then analyzed for the platelet count. The whole procedure was measured at room temperature (24– 25°C by the same investigator throughout the study).
The size and radius of the rotor vary with the centrifuge machine. Hence, relative centrifugal force values are used instead of rpm (revolutions per minute) for easy comparison. Relative centrifugal force is expressed as “g” and which is derived from the formula:
g = (1.118×10-5) RS2 where R is the radius of the rotor (in centimeters) and
S is the speed of the centrifuge in revolutions per minute (rpm)
In our study centrifuge R was 11.5 cm. Hence, for example, “g” for 900 rpm is
g = (1.118×10–5)×11.5×(900)2.
= 104.14 ~ 100.
The g values were calculated for the respective RPM and approximated to the nearest whole number (the nearest fifty or hundred):
Variation 1 - 900/1800 rpm =104g/417g
Variation 2 - 1600/3200 rpm = 330g/1329g
Variation 3- 2500/3500 rpm = 805g/1578g.
Results were analyzed using SPSS (statistical package for social sciences) version 16.0.
Results
A total of 72 samples were collected from 32 individuals comprising 28 males and four females. They were in the age group of 18–50 years. Most of the patients were in the age group of 18–25 (50%). Patterned alopecia was the most common indication (85%) followed by leg ulcers.
Platelet yield in the pure platelet-rich plasma group
Among the three centrifugation methods, the highest rise in platelet concentration was seen with 100g/400 g speeds, with a mean rise ± standard deviation of 395.4 ± 111.1% (mean platelet count attained, 8.46 lakhs per mm3), which was higher than the output of the other two methods. The difference was statistically significant with P = 0.000078 [Table 3].
| 100 g/400 g variant | 350 g/1350 g variant | 800 g/1600 g variant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean platelet count in WB (in lakh cells/mm3) | Mean platelet count in PRP (in lakh cells/mm3) | Mean platelet yield (%) | Mean platelet count in WB (in lakh cells/mm3) | Mean platelet count in PRP (in lakh cells/mm3) | Mean platelet yield (%) | Mean platelet count in WB (in lakh cells/mm3) | Mean platelet count in PRP (in lakh cells/mm3) | Mean platelet yield (%) | |
| Pure PRP method | 2.2 | 8.46 | 395.44±111.12 | 2.062 | 1.32 | 60.47±15.81 | 2.12 | 1.57 | 73.94±24.69 |
| Buffy coat method | 2.04 | 13.28 | 691.46±316.35 | 2.045 | 15.31 | 738.60±193.27 | 1.87 | 7.06 | 379.79±223.96 |
PRP: Platelet-rich plasma, WB: Whole blood
Platelet yield in leukocyte-rich platelet-rich plasma group
Among the three spin variations with buffy coat included, the 350 g/1350 g spin showed a higher percentage rise in platelet concentration from whole blood with a mean increase of 738.6 ± 193.3% in platelet concentration [Table 3]. This increase was more consistent (with less standard deviation) as compared to the other two variations. The 100 g/400 g variant also showed a mean rise of 691.5 ± 316.3% (but with a higher standard deviation) in platelet counts and the difference between the two variations was not statistically significant (P = 0.286 [independent t-test]). The mean increase in the 800/1600 g spin was 379.8 ± 224.0%. The difference between the 800/1600 g spin (lower mean increase in platelet concentration) versus the 100/400 g and 350/1350 g variations (higher values) was statistically significant of (P = 0.000459 and 0.000165, respectively).
Platelet yields between pure-platelet-rich plasma and leukocyte-rich platelet-rich plasma groups compared
When compared to pure platelet-rich plasma (leukocyte-poor platelet-rich plasma), inclusion of the buffy coat layer for the second spin (leukocyte-rich platelet-rich plasma) resulted in 174.9%, 1222.9% and 513.7% higher platelet concentrations in the 100 g/400 g, 350 g/1350 g and 800 g/1600 g spin variations, respectively [Figure 2]. This denotes that in the 100 g/400 g method 40–50% of platelets were in the buffy coat, whereas after the 350 g/1350 g spin nearly 90% of the platelets were in the buffy coat and only 10% in the plasma as seen in the lower platelet yield in the pure platelet-rich plasma method using the 350 g/1350 g spin. Overall, there was no significant difference with regard to the platelet yield between (100 g/400 g and 350 g/1350 g) speeds in the buffy coat method.
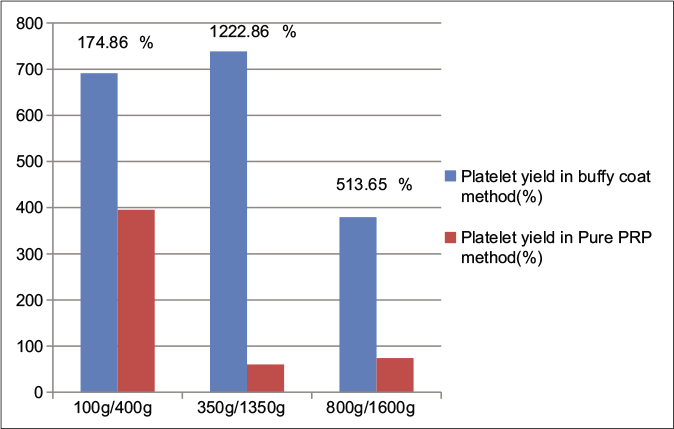
- Comparison of platelet yield between pure PRP (leukocyte-poor platelet-rich plasma) and buffy coat (leukocyte-rich platelet-rich plasma) groups at various centrifugation speeds and percentage rise of leukocyte-rich platelet-rich plasma with respect to leukocyte-poor platelet-rich plasma
Even though the buffy coat inclusion method offers higher platelet concentrations in platelet-rich plasma, it comes at the cost of higher contamination with RBCs and white blood cells (WBCs). The mean values in WBC and RBC counts with different spins, while including the buffy coat and in pure platelet-rich plasma, are given in Figures 3 and 4. An increase in the WBCs and RBCs was seen in the 100 g/400 g spin as compared to other spins in the pure platelet-rich plasma samples. A rise in platelet yield was also noted if the whole blood platelet count was <2 lakh/mm3 but the significance of this is unclear.
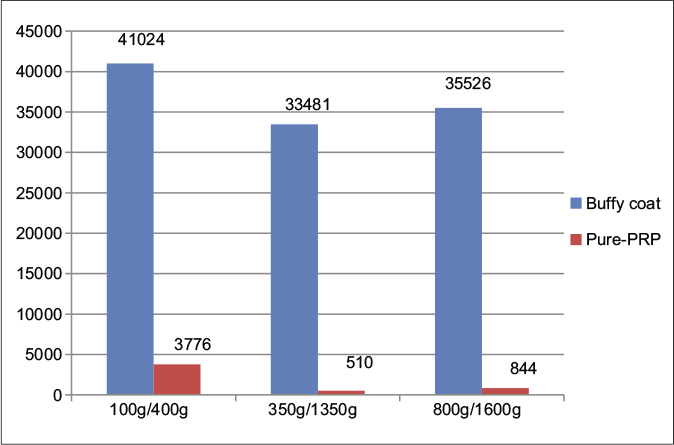
- Comparison of mean white blood cells in cells/mm3 between pure PRP (leukocyte-poor platelet-rich plasma) and buffy coat (leukocyte-rich platelet-rich plasma) groups at various centrifugation speeds
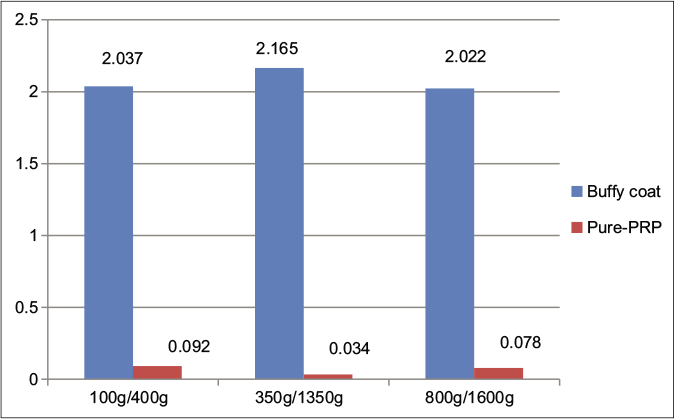
- Comparison of mean red blood cell count (in million cells/mm3) between pure PRP (leukocyte-poor platelet-rich plasma) and buffy coat (leukocyte-rich platelet-rich plasma) groups at various centrifugation speeds
Discussion
Platelet-rich plasma has been in use for quite long to accelerate wound healing and tissue repair. Its clinical application has expanded to fields such as dermatology, orthopedics, cosmetic medicine, oral and maxillofacial surgery, plastic surgery, sports medicine, cardiac surgery and ophthalmology.13 It is believed that various growth factors such as platelet-derived growth factor, transforming growth factor beta, vascular endothelial growth factor, insulin-like growth factor-1 and epidermal growth factor are released from activated platelets in platelet-rich plasma, which, in turn, promote cell proliferation, angiogenesis, chemotaxis and cell differentiation.4,14 The improved dermatologic outcomes seen are attributed to angiogenesis, neocollagenesis and adipogenesis.15 These changes are considered to be responsible for the healing and regenerative properties of platelet-rich plasma and its role in the treatment of alopecia, chronic ulcers, skin rejuvenation and various scar treatments in dermatology.
Rughetti et al. found that the platelet count and functional activity were related in a bell-shaped manner, wherein optimal stimulation for proliferation of endothelial cells and angiogenesis peaked at 1.25 × 106 and 1.5 × 106 platelets/ mL, respectively, and further increased counts had an inverse effect on proliferation.16 A very high platelet count above the baseline value is also considered disadvantageous as it may have an inhibitory effect on the healing process.17 Therefore, the general consensus is that the platelet concentration in platelet-rich plasma should be around 1 million cells/mm3.1 Considering the normal value of platelets in the blood to be 1.5–4.5 lakhs/mm3, a 2–10 times rise in platelet concentration from whole blood to platelet-rich plasma is the desirable value. Studies have shown a rise in the platelet count ranging from 3 to 7 times the baseline value after different spins to be the most effective.7-13,18-22 The comparative chart showing different centrifuge rates and its effect on platelet output is given in Table 4.7-13,18-22
| Study | Volume of WB (ml) | 1stspin | 2ndspin | Platelet count in PRP (µL) | Percentage of rise in platelet concentration (times) |
|---|---|---|---|---|---|
| Tamimi et al. | 8.5 | 160 g×10 min | 400 g×10 min | 0.632×106 | 3.52 |
| Bausset | 10 | 130 g×15 min | 250 g×15 min | - | 3.96 |
| Jo et al. | 9 | 900 g×5 min | 1500 g×15 min | 0.633×106 | 4.2 |
| Amanda et al. | 3.5 | 100 g×10 min | 400 g×10 min | 1.222×106 | 5 |
| Yin et al. | 40 | 160 g×10 min | 250 g×15 min | >1.25×106 | 5.3 |
| Amable et al. | 4.5 | 300 g×5 min | 700 g×17 min | 0.14–0.19×106 | 5.4–7.3 times |
| Araki et al. | 7.5 | 230–270 g×10 min | 2330 g×10 min | 1.896×106 | 7.4 times |
| Our study (Lr-PRP) | 4.5 | 350 g×10 min | 1350 g×10 min | 1.531×106 | 7.38 times |
| Our study (Lr-PRP) | 4.5 | 100 g×10 min | 400 g×10 min | 1.328×106 | 6.91 times |
| Our study (Lp-PRP) | 4.5 | 100 g×10 min | 400 g×10 min | 0.846×106 | 3.94 |
PRP: Platelet-rich plasma, WB: Whole blood
In our study, we attained around 4–7 times rise in platelet concentrations with the highest mean value of 738.6% rise (15.316 lakh platelets/mm3) in the 350 g/1350 g spin. This may be due to the inclusion of the buffy coat in those samples. A study on cell counts after a single centrifugation at the rate of 180 g for 15 min found that 54% of platelets were in the buffy coat and 83% of the platelet concentration was present in lower one-third of the plasma and buffy coat.23 This explains why there is greater rise in platelet concentration in leukocyte-rich-platelet-rich plasma than in pure platelet-rich plasma in our study.
Even though inclusion of the buffy coat in platelet-rich plasma preparation gives a high platelet yield, this comes along with a high number of other cells. The role of WBCs in platelet-rich plasma is still unclear. It is hypothesized that platelet-rich plasma high in leukocyte concentration provides protection from infections, contributes to angiogenesis, increases growth factor release and hypercellularity.5 Oudelaar et al. in their systematic review on blood components in platelet-rich plasma have mentioned that the concentration of vascular endothelial growth factors is significantly more in platelet-rich plasma produced by systems with higher concentrations of platelets and leukocytes than pure platelet-rich plasma kits.24
Neutrophils are the first response elements in any injury and can induce an inflammatory response, but the role of this inflammation in regeneration or degeneration is still unclear.25 Macrophages have been verified to be important in tissue turnover and regeneration. Jiang et al. 2020 in their comparison study between leukocyte-rich-platelet-rich plasma and leukocyte-poor-platelet-rich plasma in the treatment of tendinopathy observed increased expression of arginine (which is a marker of macrophage activity) in their leukocyte-rich-platelet-rich plasma group.26 They also noticed a rise in IL-10 levels which plays an important role in activating M2b macrophages. However, other studies have shown that the responses of other cells such as fibroblasts and endothelial cells do not depend on the leukocytes in the platelet-rich plasma preparation.27
Erythrocytes may represent a source of reactive oxygen species and act as an inflammatory stress inducer.28 RBCs may affect platelet function by altering pH and promoting inflammation.29 RBCs may also induce post-inflammatory hyperpigmentation due to hemosiderin deposition when used for skin rejuvenation.30 In our study, RBC contamination was relatively lower with the 350 g/1350 g spin in the pure platelet-rich plasma sample while there was no significant difference in their number among the three spin variations for the sample with the buffy coat. While RBC contamination appears unavoidable in the buffy coat preparation, some amount is detected even in the pure platelet-rich plasma sample. This contamination can be significantly reduced if the separation of buffy coat from RBCs is done with care.
Leukocyte-rich-platelet-rich plasma injection is a proven treatment for inflammatory pathologies such as lateral epicondylitis, patellar tendinopathy, plantar fasciitis and leukocyte-poor-platelet-rich plasma in osteoarthritis of knee.31 In dermatology leukocyte-rich-platelet-rich plasma has been found to successfully heal chronic diabetic ulcers, small venous ulcers, complex multifactorial wounds, pressure ulcers and leprosy ulcers.6,32-36 A study conducted by Setta et al. on chronic diabetic ulcers of 12 weeks ulcer duration found that using leukocyte-rich-platelet-rich plasma gel led to a shorter mean healing time (11.5 weeks) as compared to leukocyte-poor-platelet-rich plasma (17 weeks).36 Anandan et al. found that 92% of patients with leprosy ulcers showed complete re-epithelialization within six weeks after weekly treatment sessions with leukocyte-rich-platelet-rich plasma.34 Both leukocyte-rich-platelet-rich plasma and leukocyte-poor-platelet-rich plasma are known to work in acne scars.37,38 Addition of leukocyte-rich-platelet-rich plasma to laser ablation produced additional benefits in acne scarring and also improved symptoms such as erythema, edema and pain.39
On the other hand, leukocytes may release pro-inflammatory cytokines such as tumor necrosis factor-alpha, IL-6 and nuclear factor kappa-b mediated activated B-cell pathways, which may increase inflammation and promote degenerative changes.40 This may negate the inclusion of the buffy coat to increase the platelet count in conditions where there is a preexisting inflammatory pathology such as vitiligo or ulcers. Ibrahim et al. evaluated fortnightly treatment with leukocyte-poor-platelet-rich plasma in stable vitiligo for four months and found that 75% repigmentation was achieved in 55% of the lesions.41 However, since there are no comparative studies between leukocyte-rich-platelet-rich plasma and leukocyte-poor-platelet-rich plasma in ulcer treatment or in vitiligo; it is difficult to assess the superiority of either treatment modality.
A study using leukocyte-poor-platelet-rich plasma by Ibrahim et al. on scars due to acne, trauma or varicella showed that non-acne scars responded better than acne scars. In acne scars a good improvement was noted when platelet-rich plasma was combined with micro needling (70.4%).42
In androgenic alopecia, the positive or negative effects of leukocytes in platelet-rich plasma are debatable. Both leukocyte-rich-platelet-rich plasma and leukocyte-poor-platelet-rich plasma have shown good efficacy in androgenic alopecia.14,43-45 A study of leukocyte-rich-platelet-rich plasma in androgenic alopecia by Schiavone et al. showed improvements in global physician assessment score in most of the participants.45
Limitations
The study was limited by a small sample size in the pure platelet-rich plasma (leukocyte-poor-platelet-rich plasma) group. Analysis of platelet yield through automated platelet-rich plasma systems was not done. Further analysis will be required to compare the efficacy of leukocyte-rich-platelet-rich plasma and leukocyte-poor-platelet-rich plasma in various dermatological indications.
Conclusion
Our study showed that in our setting, an ideal platelet yield in platelet-rich plasma can be achieved with both the 100 g/400 g as well as the 350 g/1350 g spins while using the buffy coat (leukocyte-rich-platelet-rich plasma), and the 100 g/400 g spins method for pure platelet-rich plasma (leukocyte-poor-platelet-rich plasma) accomplishes a near-ideal platelet count along with low contamination with other cells.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001;10:225-8.
- [CrossRef] [PubMed] [Google Scholar]
- Preoperative plasmapheresis in patients undergoing cardiac surgery procedures. Anesthesiology. 1990;72:282-8.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma biology In: Alves R. Grimalt R, editors. Clinical Indications and Treatment Protocols with Platelet-Rich Plasma in Dermatology. Barcelona: Ediciones; 2016. p. :3-15.
- [Google Scholar]
- Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011;23:424-31.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158-67.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma and its utility in medical dermatology: A systematic review. J Am Acad Dermatol. 2019;81:834-46.
- [CrossRef] [PubMed] [Google Scholar]
- Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. Int Sch Res Notices. 2014;2014:176060.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67.
- [CrossRef] [PubMed] [Google Scholar]
- Optimum centrifugation conditions for the preparation of platelet and plasma products. Transfusion. 1976;16:162-5.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. 1976;34:395-402.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300.
- [CrossRef] [Google Scholar]
- Optimizing platelet-rich plasma gel formation by varying time and gravitational forces during centrifugation. J Oral Implantol. 2013;39:525-32.
- [CrossRef] [PubMed] [Google Scholar]
- Optimized preparation method of platelet-concentrated plasma and noncoagulating platelet-derived factor concentrates: Maximization of platelet concentration and removal of fibrinogen. Tissue Eng Part C Methods. 2012;18:176-85.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491-7.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma for the aesthetic surgeon. Facial Plast Surg. 2017;33:437-43.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet gel-released supernatant modulates the angiogenic capability of human endothelial cells. Blood Transfus. 2008;6:12-7.
- [Google Scholar]
- Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665-71.
- [CrossRef] [PubMed] [Google Scholar]
- Formulation and storage of platelet-rich plasma homemade product. Biores Open Access. 2012;1:115-23.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of 2 methods for obtaining platelet-rich plasma. J Oral Maxillofac Surg. 2007;65:1084-93.
- [CrossRef] [PubMed] [Google Scholar]
- Optimization of pure plateletrich plasma preparation: A comparative study of pure platelet-rich plasma obtained using different centrifugal conditions in a single-donor model. Exp Ther Med. 2017;14:2060-70.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of autologous preparation rich in growth factors for the treatment of chronic cutaneous ulcers. J Biomed Mater Res B Appl Biomater. 2008;84:415-21.
- [CrossRef] [PubMed] [Google Scholar]
- Optimized centrifugation preparation of the platelet rich plasma: Literature review. J Stomatol Oral Maxillofac Surg. 2020;121:150-4.
- [CrossRef] [PubMed] [Google Scholar]
- An important and overlooked parameter in platelet rich plasma preparation: The mean platelet volume. J Cosmet Dermatol. 2019;18:474-82.
- [CrossRef] [PubMed] [Google Scholar]
- Concentrations of blood components in commercial platelet-rich plasma separation systems: A review of the literature. Am J Sports Med. 2019;47:479-87.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of leukocyte-rich platelet-rich plasma and leukocyte-poor platelet-rich plasma on achilles tendinopathy at an early stage in a rabbit model. Am J Sports Med. 2020;48:1189-99.
- [CrossRef] [PubMed] [Google Scholar]
- Leukocyte depletion does not affect the in vitro healing ability of platelet rich plasma. Exp Ther Med. 2018;15:4029-38.
- [CrossRef] [PubMed] [Google Scholar]
- DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc Med. 2016;2:e000060.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of platelet-rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am J Sports Med. 2014;42:1204-10.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma increases pigmentation. J Craniofac Surg. 2017;28:e793.
- [CrossRef] [PubMed] [Google Scholar]
- Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-34.
- [CrossRef] [PubMed] [Google Scholar]
- Leucocyte-and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets. 2018;29:468-75.
- [CrossRef] [PubMed] [Google Scholar]
- Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J Cosmet Dermatol. 2018;17:495-501.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet rich plasma: Efficacy in treating trophic ulcers in leprosy. J Clin Diagn Res. 2016;10:WC06-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and safety of autologous platelet-rich plasma therapy with total contact casting versus total contact casting alone in treatment of trophic ulcer in leprosy: An observer-blind, randomized controlled trial. Indian J Dermatol Venereol Leprol. 2020;86:262-71.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int Wound J. 2011;8:307-12.
- [CrossRef] [PubMed] [Google Scholar]
- Combined autologous platelet-richplasma with microneedling verses microneedling with distilledwater in the treatment of atrophic acne scars: A concurrent split-face study. J Cosmet Dermatol. 2016;15:434-43.
- [CrossRef] [PubMed] [Google Scholar]
- Skin microneedling plusplatelet-rich plasma versus skin microneedling alone in thetreatment of atrophic post acne scars: A split face comparativestudy. J Dermatolog Treat. 2018;29:281-6.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma and its utility in the treatment of acne scars: A systematic review. J Am Acad Dermatol. 2019;80:1730-45.
- [CrossRef] [PubMed] [Google Scholar]
- The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
- [CrossRef] [PubMed] [Google Scholar]
- Theeffect of platelet-rich plasma on the outcome of short-termnarrowbandeultraviolet B phototherapy in the treatment of vitiligo: A pilot study. J Cosmet Dermatol. 2016;15:108-16.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic effect of microneedling and autologous platelet-rich plasma in the treatment of atrophic scars: A randomized study. J Cosmet Dermatol. 2017;16:388-99.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int J Womens Dermatol. 2019;5:46-51.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci. 2015;9:159-62.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma for androgenetic alopecia: A pilot study. Dermatol Surg. 2014;40:1010-9.
- [CrossRef] [PubMed] [Google Scholar]






