Translate this page into:
A cross-sectional study of the histopathology and immunology of alopecia areata: Unearthing the role of the Janus kinase–signal transducer and activator of transcription pathway
2 Department of Pathology, Maulana Azad Medical College, Govind Ballabh Pant Hospital, New Delhi, India
Correspondence Address:
Vineet Relhan
Department of Dermatology, Maulana Azad Medical College, Bahadur Shah Zafar Marg, New Delhi - 110 002
India
| How to cite this article: Bansal A, Relhan V, Garg VK, Saran RK. A cross-sectional study of the histopathology and immunology of alopecia areata: Unearthing the role of the Janus kinase–signal transducer and activator of transcription pathway. Indian J Dermatol Venereol Leprol 2019;85:455-461 |
Abstract
Background: Alopecia areata is an autoimmune disease that occurs as a result of the loss of the inherent immune privilege of the hair follicle. It has been recently demonstrated that the interferon-γ/interleukin-15 feedback loop that signals via the Janus kinase–signal transducer and activator of transcription pathway is critical to the breakdown of this immune privilege.
Aims: To evaluate the immunological distribution of CD4+ T-cells, CD8+ T-cells, phosphorylated signal transducer and activator of transcription 1 and study its relation with the clinical and histopathological findings of the disease.
Materials and Methods: A total of 30 patients of alopecia areata were included in the study. Following a detailed history and clinical examination, a scalp biopsy was performed. Histopathology was studied and immunohistochemistry was done to demonstrate the positivity and distribution of CD4+ T-cells, CD8+ T-cells and phosphorylated signal transducer and activator of transcription 1.
Results: The follicular count, number of anagen and terminal hair were found to be decreased, whereas the catagen, telogen and vellus hair were found to be increased in number. A peribulbar CD4+ T-cell infiltrate was seen in 70% cases, whereas a CD8+ T-cell infiltrate was seen in 83.3% cases. An intrabulbar CD4+ T-cell infiltrate was seen in 26.7% cases, whereas a CD8+ T-cell infiltrate was seen in 70% cases. Among the 25 hair follicles dermal papilla identified, 36.8% cases were found to be positive for phospho-signal transducer and activation of transcription-1.
Limitations: The drawbacks of our study included a small sample size and the use of only vertical sectioning for the scalp biopsy samples.
Conclusion: Phosphorylated signal transducer and activator of transcription 1 positivity as an indicator of signalling via the Janus kinase-1/2 pathway was seen in 36.8% of our cases highlighting the integral role of this pathway in the pathogenesis of alopecia areata.
Introduction
Alopecia areata is a noncicatricial alopecia with an incidence of 0.1–0.2% in the world with an equal preponderance in males and females.[1],[2] It is most commonly reported on the scalp, typically as a smooth, round, bald patch, while the complete loss of scalp hair (alopecia totalis) or the complete loss of scalp and body hair (alopecia universalis) have also been reported.[2],[3] Alopecia areata is considered to be a hair-specific autoimmune disease, characterized by a T-cell-mediated inflammation of the anagen hair. Among genetically predisposed individuals, this happens as a result of the collapse of its immune privilege, mediated by interferon-γ. This leads to major histocompatibility complex class I expression in the proximal hair bulb, thus expressing self-epitopes to cytotoxic CD8+ T-cells, which subsequently enter the hair follicle. CD4+ T-cells constitute the majority of lymphocytes in the perifollicular area.[4],[5] This peribulbar and intrabulbar lymphocytic infiltrate around anagen follicles, resembling a “swarm of bees”, characteristic of alopecia areata.[6]
Recently, the role of CD8+ natural killer, group 2, member D protein (NKG2D) T-cells and the interferon-γ/interleukin-15 feedback loop has come into the picture.[7] These function via the Janus kinase–signal transducer and activator of transcription pathway. Interferon-γ released from these activated T-cells leads to signal transducer and activator of transcription-1 activation, causing it's translocation to the nucleus, which leads to interleukin-15 production.[7] Interleukin-15 enhances the production of interferon-γ, thus perpetuating this critical feedback loop.[7]
We undertook this study to elucidate the clinical and histopathological findings of this disease process and understand the role of the Janus kinase–signal transducer and activator of transcription pathway in the pathogenesis of this disease process via demonstration of phosphorylated transducer and activator of transcription-1 in the hair follicles of patients with alopecia areata. There are very few studies that have evaluated this aspect, hence we decided to undertake this study.
Materials and Methods
This was a cross-sectional study carried out between October 2015 and March 2017. Thirty patients of alopecia areata presenting to the outpatient clinic of the Department of Dermatology, Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi were recruited. Ethical approval was obtained from Institutional Review Board vide letter IRBNo. IEC/MAMC/2015/717.
Pregnant or lactating females; patients with history of intake and/or application of corticosteroids in any form within last 1 month were excluded from the study.
A detailed history was obtained and clinical examination done and recorded on predesigned proformas. Severity of hair loss was assessed using the Severity of Alopecia Tool Score. The percentage of scalp hair loss in both sides, back and top of the scalp were determined independently. Each value was multiplied by the % scalp covered in that area of the scalp and the products of each section summed for a final total % hair loss, designated as the SALT score with the following categories: S0 = no hair loss; S1≤ 25% hair loss; S2= 25–49% hair loss; S3= 50–74% hair loss; S4= 75–99% hair loss; S5= 100% hair loss.[8]
A biopsy was performed under local anesthesia from the margin of the lesion, using a 4 mm punch for the purpose of histopathological and immunological evaluation. The tissue was placed in 10% buffered formalin and processed for histopathology by routine paraffin embedding in a vertical orientation. Then, 3–5 μm sections were cut and stained with hematoxylin and eosin. Immunohistochemistry was done on the unstained sections using the antibodies against CD4 (monoclonal mouse antihuman CD4, DAKO, ready to use) CD8 (monoclonal mouse antihuman CD8, Thermoscientific, ready to use) and phospho-signal transducer and activation of transcription-1 (Tyr701) (58D6). Intrabulbar and peribulbar CD4+ T-cells and CD8+ T-cells were identified and the grading was done, as shown in [Table - 1].[9],[10] Phosphorylated transducer and activator of transcription-1 nuclear staining was assessed in the hair follicle dermal papilla and graded as shown in [Table - 1].

The primary outcome measures were the number of hair follicle dermal papillae demonstrating phosphorylated transducer and activator of transcription-1 positivity and the number of hair follicles showing CD4+/CD8+ T-cells in the peribulbar and intrabulbar location based on immunohistochemistry.
The secondary outcome measures were the relations between the immunological pattern of CD4+ T-cells, CD8+ T-cells, phosphorylated transducer and activator of transcription-1 with the clinical (disease duration, pattern and severity) and histopathological (lymphocytic infiltrate) parameters.
The quantitative variables were expressed as mean ± SD and compared between groups using Kruskal–Wallis test, unpaired t-test and Mann–Whitney U-test wherever applicable. Also, qualitative variables were expressed as frequencies/percentages and compared between groups using Chi-square test and Fisher's exact test wherever applicable. Data were analyzed using Statistical Packages for the Social Sciences, and a P value < 0.05 was considered significant.
Results
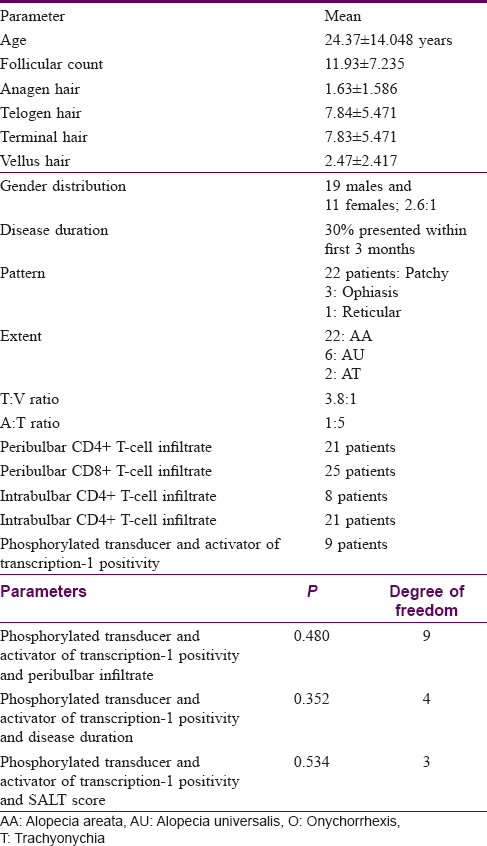
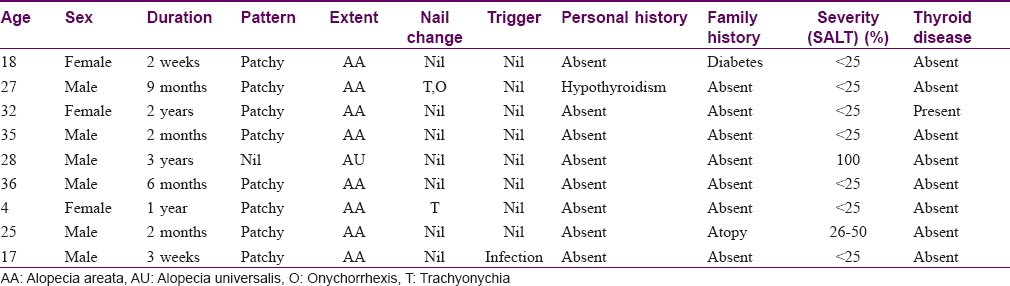
The study population comprised 30 patients, 19 males and 11 females. The distribution of age in our study ranged from 4 years to 57 years with the mean age of 24.37 ± 14.048 years. The duration of the disease in our study ranged from 2 days to 8 years, with the maximum number of patients (30%) presenting within the first 3 months of disease. In our study, 22 patients (73.3%) presented with alopecia areata, whereas 6 (20%) had alopecia universalis and 2 (6.7%) had alopecia totalis. Among the 22 patients having patchy hair loss, 4 (13.3%) had patterned hair loss. Out of these, 3 (10%) patients presented with an ophiasis pattern and 1 (3.3%) patient with a reticular pattern.
In our study, the follicular count ranged from 3 to 32, with a mean follicular count of 11.93 ± 7.235. The mean number of anagen hair were 1.63 ± 1.586, and the mean number of telogen hair were 7.84 ± 5.471. Mean number of terminal hair were 7.83 ± 5.471 and mean vellus hair were 2.47 ± 2.417. The terminal hair to vellus hair ratio was 3.8:1 and anagen to telogen hair ratio was 1:5.
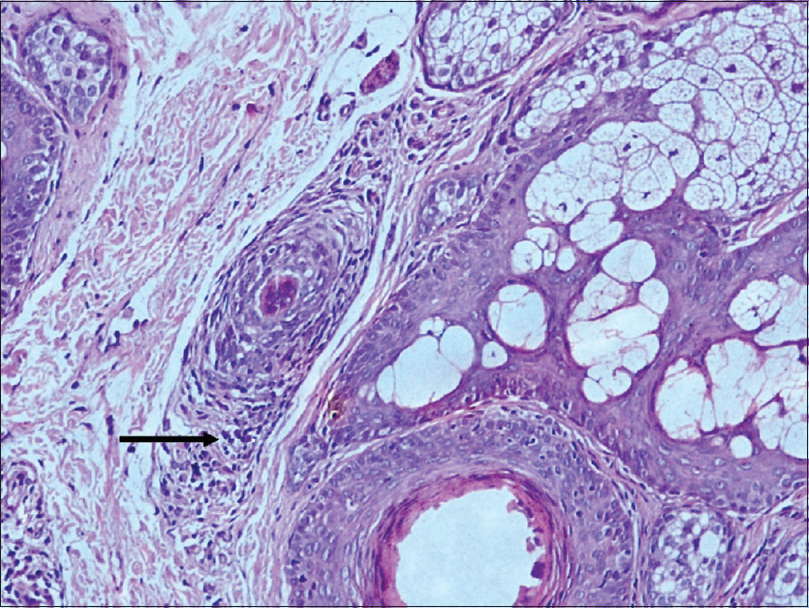
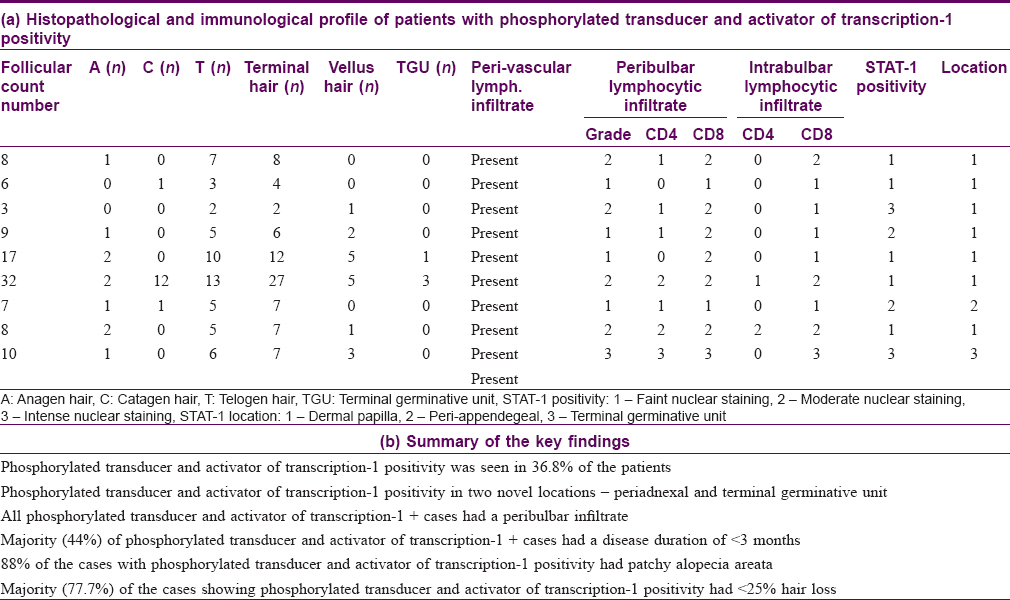
A peribulbar lymphocytic infiltrate was identified in 27 (90%) of the cases [Figure - 1] and [Figure - 2]. A peribulbar CD4+ T-cell infiltrate was seen in 21 (70%) cases, whereas a CD8+ T-cell infiltrate was seen in 25 (83.3%) cases. An intrabulbar CD4+ T-cell infiltrate was seen in 8 (26.7%) cases, whereas a CD8+ T-cell infiltrate was seen in 21 (70%) cases [Figure - 3] and [Figure - 4]. From the 30 cases in our study, hair follicle dermal papilla could be identified in the section in 19 cases, out of which 7 (36.8%) cases were positive for Phosphorylated transducer and activator of transcription-1. Positivity in two other locations – terminal germinative unit and periadnexal area were seen in two additional cases. The grade of staining was faint in five cases, moderate in two cases and intense in two cases [Figure - 5].
 |
| Figure 1: “Swarm of bees:” Peribulbar lymphocytic infiltrate: Anagen hair follicle [hematoxylin and eosin (×100)] |
 |
| Figure 2: Peribulbar lymphocytic infiltrate around a miniaturized hair follicle (H and E, ×100) |
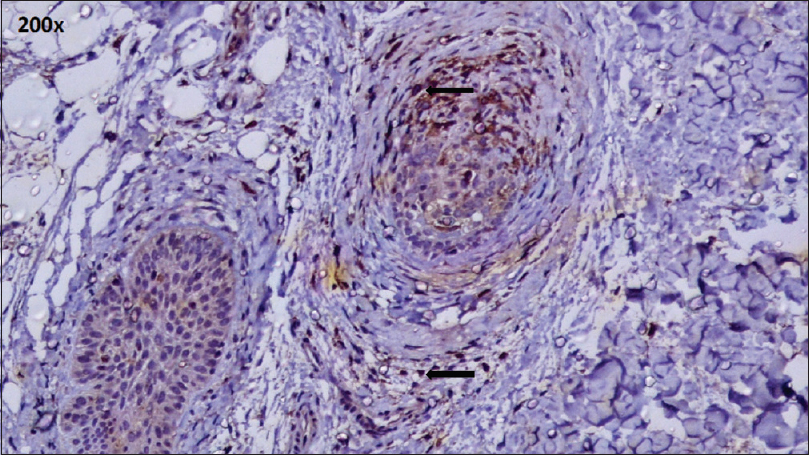 |
| Figure 3: CD4+ T cell infiltrate: Peribulbar and intrabulbar (IHC, ×200) |
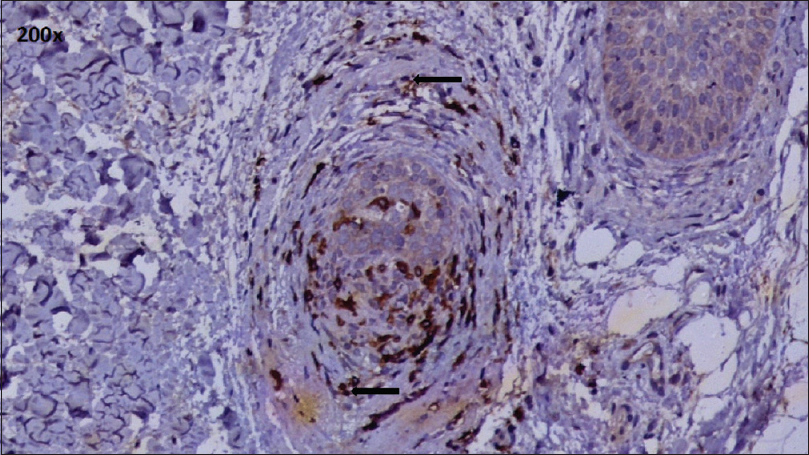 |
| Figure 4: CD8+ T cell infiltrate: Peribulbar and intrabulbar(IHC, ×200) |
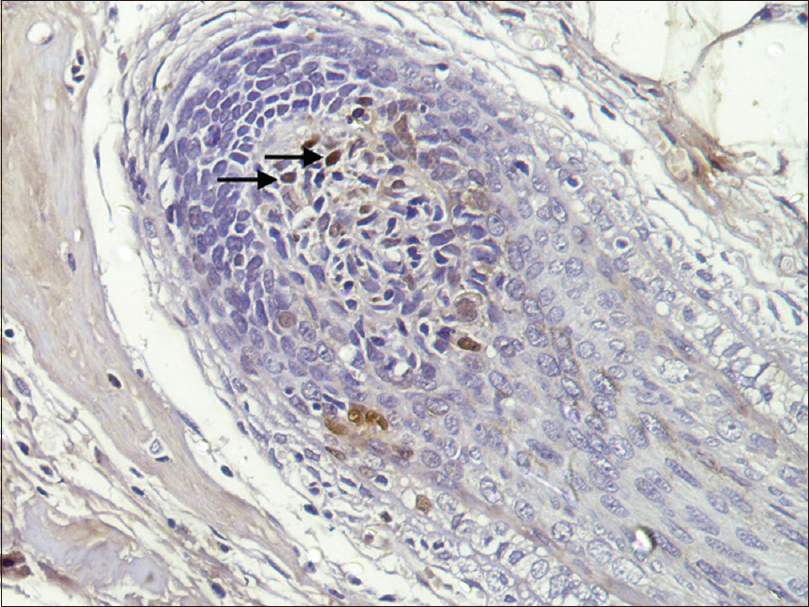 |
| Figure 5: Phosphorylated transducer and activator of transcription 1 positivity: Hair follicle dermal papilla (IHC, ×400) |
A peribulbar infiltrate was identified in 27 cases and out of these 9 (33.3%) cases were positive for Phosphorylated transducer and activator of transcription-1. None of the cases without a peribulbar infiltrate showed signal transducer and activator of transcription-1 positivity. This association was not found to be statistically significant (P value = 0.480).
Phosphorylated transducer and activator of transcription-1 positivity was seen in 4 out of 9 (44.4%) patients with a disease duration of <3 months, 2 out of 7 cases (28.5%) with a disease duration of 3–12 months and 2 out of 4 cases (50%) with a disease duration of 12–24 months, while it was seen in 1 out of 4 cases (25%) with a disease duration between 2 and 5 years, and none out of the six cases with a disease duration of >5 years. However, the association between phospho-signal transducer and activation of transcription-1 positivity with the disease duration was not found to be statistically significant (P value = 0.352) Phosphorylated transducer and activator of transcription-1 was positive in 8 out of the 22 (36.3%) alopecia areata (patchy) cases and 1 out of 6 (16.6%) alopecia universalis cases, and none of the alopecia totalis cases.
Phosphorylated transducer and activator of transcription-1 positivity was seen in 7 of 17 cases that had < 25% hair loss, 1 of 4 cases with 25–50% hair loss and 1 of 8 cases with 100% hair loss. This association was not found to be statistically significant (P value = 0.534).
These findings have been summarized in [Table - 2], [Table - 3], [Table - 4].
Discussion
Alopecia areata is a non-cicatricial alopecia caused by a T-cell-mediated autoimmune attack as a result of the immune privilege collapse of the anagen hair follicle.[3] Male preponderance was observed in our study with 19 (63%) male and 11 (27%) female patients with a male to female ratio of 2.6:1, which is in accordance with the findings noted in other studies from India.[1],[11] In our study, 16 (53%) patients presented within 1 year of disease onset. In a study by Jain et al., 78% patients presented within 6 months of disease onset. The majority of our patients (73.3%) presented with patchy disease pattern, similar to various studies that report patchy alopecia areata as the most common (75%) pattern in the world,[4] as well as in India.[11],[12]
The mean follicular count was found to be 11.92 in our study, similar to findings that have been reported in various studies where the follicular count in alopecia areata has been found to be reduced by 25% from the normal count of 30–40.[13],[14]
The anagen hair were found to be decreased, with a mean number of 1.63. There was an increase in the nonanagen hair – catagen and telogen hair, with the anagen: telogen ratio being 1:4.1.
Vellus hair were found to be increased and terminal hair decreased, with a terminal to vellus ratio of 3.8:1. Other studies report similar findings with a decrease in the anagen count, increase in the nonanagen hair, increase in the vellus hair and a decrease or reversal of the A:T and T:V ratio. Marwah et al.[15] report a T:V ratio of 1:3.6; Chaitra et al.[16] report an A: T ratio of 1:1.62.
With the collapse of the immune privilege in alopecia areata, CD8+ cells enter the hair follicle, whereas CD4+ T-cells constitute the majority of lymphocytes in the perifollicular area.[5],[6]
A peribulbar lymphocytic infiltrate was identified in 27 (90%) of our cases, whereas Marwah et al. reported it in 76% of their cases.[15]
A peribulbar CD4+ T-cell infiltrate was seen in 21 (70%) cases, whereas a CD8+ T-cell infiltrate was seen in 25 (83.3%) cases. Most of the studies report that the peribulbar infiltrate is predominantly CD4+ T-cell in nature with CD4:CD8 ratios varying from 1.1:1 to 2.3:1.[17],[18] CD4:CD8 ratio has been shown to vary based on the disease activity,[19] severity [20] and pattern,[17] and the findings in our study could be because of an over-representation of severe disease in the sample because CD4:CD8 ratio decreases with increasing disease severity.[20] However, Xing et al.[7] have reported CD8+ T-cells as the predominant cell type and found CD4 T-cells to be infrequent in alopecia areata lesional skin.
In our study, the intrabulbar infiltrate was composed of CD8+ T-cells among 21 (70%) of the cases, similar to the findings of Todes-Taylor et al.[19] and Ranki et al.[18]
Recently, studies with mouse and human disease models have brought into picture the critical role of the CD8+ NKG2D T-cells and the interferon-γ/interleukin-15 feedback loop in the pathogenesis of alopecia areata. It has been demonstrated that CD8+ NKG2D T-cells enter the dermis, surround the hair follicle bulb, binding to the major histocompatibility complex-I peptide complex and NKG2D ligand present on the hair follicle epithelial cells. These activated T-cells now produce interferon-γ which binds to its receptor on the follicular epithelial cells, activates Janus kinase-1/Janus kinase-2 which causes signal transducer and activator of transcription-1 which is translocated to the nucleus leading to interleukin-15 production. Interleukin-15 now binds to its receptor complex on the CD8+ T cells, and causes Janus kinase-1/Janus kinase-3 as well as signal transducer and activation of transcription-5, enhancing the production of interferon-γ, thus perpetuating this critical feedback loop [Figure - 6].[7] Recently, this interferonγ signaling through Janus kinase-1/2 has been demonstrated by the presence of phosphorylated signal transducer and activator of transcription proteins (phosphorylated transducer and activator of transcription-1, phosphorylated transducer and activator of transcription-3) in human and mouse alopecic hair follicles, but not in normal hair follicles.[7]
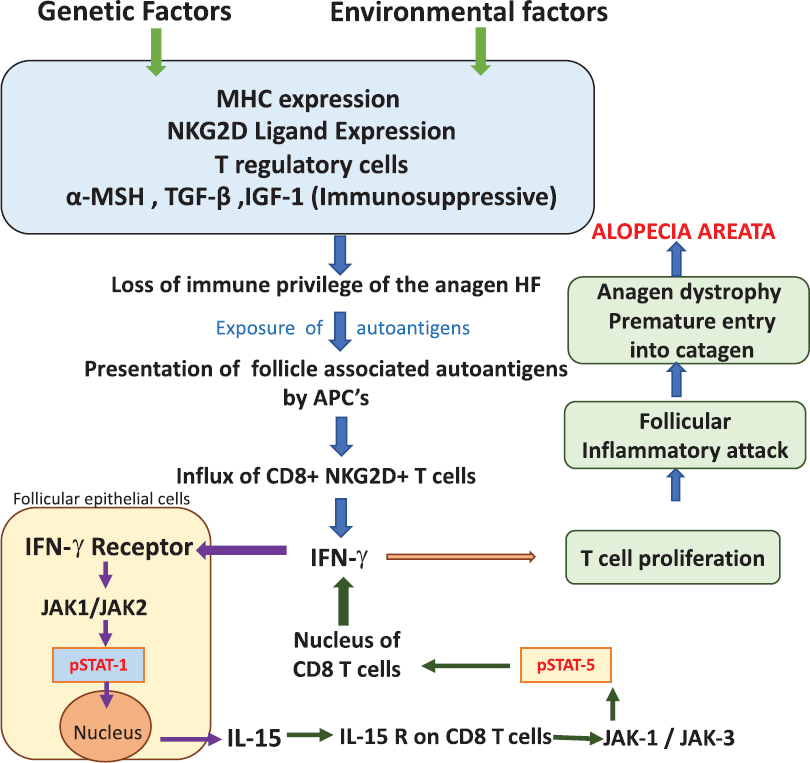 |
| Figure 6: Critical role of the CD8+ NKG2D T cells and the interferon-γ/interleukin-15 feedback loop in the pathogenesis of alopecia areata. CD8 + NKG2D T cells enter the dermis, surround the hair follicle bulb, bind to the major histocompatibility complex-I peptide complex and NKG2D ligand present on the hair follicle epithelial cells. These activated T cells now produce interferon-γ which binds to its receptor on the follicular epithelial cells, activates Janus kinase-1/Janus kinase-2 which further cause signal transducer and activator of transcription-1 activation, which is translocated to the nucleus leading to interleukin-15 production. Interleukin-15 now binds to its receptor complex on the CD8+ T-cells and causes Janus kinase-1/Janus kinase-3 as well as signal transducer and activator of transcription-5 activation, enhancing the production of interferon-γ, thus perpetuating this critical feedback loop |
In our study, using immunohistochemistry we found phosphorylated transducer and activator of transcription-1 positivity in nine of our cases.
Out of the 30 vertical sections assessed, hair follicle dermal papillae were identified in 19 cases. Phosphorylated transducer and activator of transcription-1 positivity was found in the hair follicle dermal papilla cells in 7 (36.8%) cases, whereas positivity in two other novel locations – terminal germinative unit and periadnexal area was seen in one case each. The reduced hair follicle dermal papilla count in our study may be because of a 25% reduction in follicle numbers that has been reported in alopecia areata, from the normal follicular count of 30–40 hair per 4 mm biopsy.[13],[14] However, these values have been reported from studies done in a Western population, and a lesser hair density has been shown among Asians.[21] Furthermore, with vertical sectioning, as was done in our study, only 10–15% of the sample follicles are visualized; therefore several serial sections [22] and multiple biopsies may be necessary [23] to maximize the diagnostic yield, which was not feasible in our set up owing to a limitation of resources and ethical concerns.
In a study done by Xing et al., patients fulfilling the following criteria were selected: 30–95% hair loss owing to alopecia areata, hair loss duration of at least 3 months; subject age 18–75 years.[7] Phosphorylated transducer and activator of transcription-1 positivity was seen in five patients using Western-blot analysis and percentage positivity was not elaborated. However, in our study in all patients of alopecia areata irrespective of age, disease duration and percentage of hair loss, an immunohistochemical analysis was done. It remains to be elucidated on the extent to which these factors might have further affected the phosphorylated transducer and activator of transcription-1 positivity. We also made an attempt to correlate the presence of phosphorylated transducer and activation of transcription-1 with the other available parameters. In our study none of the cases without a peribulbar infiltrate showed signal transducer and activator of transcription-1 positivity, but this association was not found to be statistically significant (P value = 0.480).
A large number of cases, i.e. 4 out of 9 cases (44%), demonstrating phosphorylated transducer and activator of transcription-1 positivity presented with an acute onset of disease (<3 months duration). In our study, 8 out of 9 (88%) cases with phosphorylated transducer and activation of transcription-1 positivity had localized disease (alopecia areata), while only one patient with alopecia universalis demonstrated phosphorylated transducer and activation of transcription-1 positivity. These findings were similar to those of Xing et al. where all the cases with phosphorylated transducer and activator of transcription-1 positivity were alopecia areata and no alopecia totalis or alopecia universalis case was included in the study.[7]
A majority of our patients, i.e. 7 out of 9 (77.7%), with phosphorylated transducer and activator of transcription-1 positivity had < 25% hair loss, whereas only one case each belonged to the 25–50% and 100% hair loss categories. Xing et al. included only patients with 30–95% hair loss in their study and found no association with disease extent or severity. Thus, further research is required to study if this positivity has any association with extent of disease.[7]
Limitations
The limitations of our study, as mentioned earlier, included a small sample size, performing a single biopsy and the use of only vertical sectioning which may have adversely affected the detection of phosphorylated transducer and activator of transcription-1 positivity.
Conclusion
The present study assessed the clinical features, histopathology and immunology of alopecia areata. A peribulbar lymphocytic infiltrate was identified in majority of our cases which was composed of both CD4+ and CD8+ T-cells while the intrabulbar infiltrate was composed predominantly of CD8+ T-cells.
Phosphorylated transducer and activator of transcription-1 positivity as an indicator of Janus kinase-1/2 signaling pathway was seen in 7 (36.8%) of our cases, demonstrating the critical role the role of this pathway in the pathogenesis of alopecia areata.
Furthermore, we propose that phosphorylated transducer and activator of transcription-1 positivity may be an indicator of acute, progressive disease with an active CD8 T-cell predominant peribulbar inflammatory infiltrate indicative of a possible good treatment response.
With many recent studies suggesting that pharmacological inhibition of this pathway via Janus kinase-signal transducer and activator of transcription inhibitors such as ruxolitinib and tofacitinib leads to restoration of hair growth in alopecia areata, the understanding of this critical signaling pathway has become even more relevant today.[24]
However, further studies with a larger sample size are needed to clearly establish the role of the Janus kinase–signal transducer and activator of transcription pathway in the pathogenesis of alopecia areata.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol 2007;46:121-31.
[Google Scholar]
|
| 2. |
Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int J Dermatol 1996;35:22-7.
[Google Scholar]
|
| 3. |
Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol 2000;42:549-66.
[Google Scholar]
|
| 4. |
Norris DA. Genes and immune response in alopecia areata: Review of the alopecia areata research summit first day proceedings. J Investig Dermatol Symp Proc 2013;16:S10-2.
[Google Scholar]
|
| 5. |
Wang E, McElwee KJ. Etiopathogenesis of alopecia areata: Why do our patients get it? Dermatol Ther 2011;24:337-47.
[Google Scholar]
|
| 6. |
Seetharam KA. Alopecia areata: An update. Indian J Dermatol Venereol Leprol 2013;79:563-75.
[Google Scholar]
|
| 7. |
Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014;20:1043-9.
[Google Scholar]
|
| 8. |
Olsen E, Hordinsky M, McDonald-Hull S, Price V, Roberts J, Shapiro J, et al. Alopecia areata investigational assessment guidelines. National alopecia areata foundation. J Am Acad Dermatol 1999;40:242-6.
[Google Scholar]
|
| 9. |
Sato M, Amagai M, Ohyama M. Detailed clinicopathological characterization of progressive alopecia areata patients treated with i.v. corticosteroid pulse therapy toward optimization of inclusion criteria. J Dermatol 2014;41:957-63.
[Google Scholar]
|
| 10. |
Bodemer C, Peuchmaur M, Fraitaig S, Chatenoud L, Brousse N, De Prost Y. Role of cytotoxic T cells in chronic alopecia areata. J Invest Dermatol 2000;114:112-6.
[Google Scholar]
|
| 11. |
Panda M, Jena M, Patro N, Dash M, Jena AK, Mishra S. Clinico-epidemiological profile and therapeutic response of alopecia areata in a tertiary care teaching hospital. J Pharm Sci Res 2014;6:169-74.
[Google Scholar]
|
| 12. |
Jain S, Marfatia YS. Alopecia areata – Pattern in industrial city of Baroda. Indian J Dermatol Venereol Leprol 2003;69:81-2.
[Google Scholar]
|
| 13. |
Whiting DA. The histopathology of alopecia areata in vertical and horizontal sections. Dermatol Ther 2001;14:297-305.
[Google Scholar]
|
| 14. |
Tobin DJ. Morphological analysis of hair follicles in alopecia areata. Microsc Res Tech 1997;38:443-51.
[Google Scholar]
|
| 15. |
Marwah M, Nadkarni N, Patil S. 'Ho-ver'ing over alopecia areata: Histopathological study of 50 cases. Int J Trichology 2014;6:13-8.
[Google Scholar]
|
| 16. |
Chaitra V, Rajalakshmi T, Kavdia R. Histopathologic profile of alopecia areata in Indian patients. Int J Trichology 2010;2:14-7.
[Google Scholar]
|
| 17. |
Zhao Y, Zhang B, Caulloo S, Chen X, Li Y, Zhang X. Diffuse alopecia areata is associated with intense inflammatory infiltration and CD8+ T cells in hair loss regions and an increase in serum IgE level. Indian J Dermatol Venereol Leprol 2012;78:709-14.
[Google Scholar]
|
| 18. |
Ranki A, Kianto U, Kanerva L, Tolvanen E, Johansson E. Immunohistochemical and electron microscopic characterization of the cellular infiltrate in alopecia (areata, totalis, and universalis). J Invest Dermatol 1984;83:7-11.
[Google Scholar]
|
| 19. |
Todes-Taylor N, Turner R, Wood GS, Stratte PT, Morhenn VB. T cell subpopulations in alopecia areata. J Am Acad Dermatol 1984;11:216-23.
[Google Scholar]
|
| 20. |
McElwee KJ, Freyschmidt-Paul P, Hoffmann R, Kissling S, Hummel S, Vitacolonna M, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(-) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol 2005;124:947-57.
[Google Scholar]
|
| 21. |
Lee HJ, Ha SJ, Lee JH, Kim JW, Kim HO, Whiting DA. Hair counts from scalp biopsy specimens in Asians. J Am Acad Dermatol 2002;46:218-21.
[Google Scholar]
|
| 22. |
Headington JT. Transverse microscopic anatomy of the human scalp. A basis for a morphometric approach to disorders of the hair follicle. Arch Dermatol 1984;120:449-56.
[Google Scholar]
|
| 23. |
Elston DM, Ferringer T, Dalton S, Fillman E, Tyler W. A comparison of vertical versus transverse sections in the evaluation of alopecia biopsy specimens. J Am Acad Dermatol 2005;53:267-72.
[Google Scholar]
|
| 24. |
Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv 2015;1:e1500973.
[Google Scholar]
|
Fulltext Views
6,003
PDF downloads
2,746





