Translate this page into:
A huge reddish brown nodulo-plaque on the face
Corresponding author: Dr. Yan Yu, Department of Dermatology, First Hospital of Jilin University, Changchun, Jilin, China. yu_yan@jlu.edu.cn
-
Received: ,
Accepted: ,
How to cite this article: Shi Y, Shan LS, Yu Y. A huge reddish brown nodulo-plaque on the face. Indian J Dermatol Venereol Leprol. 2024;90:136. doi: 10.25259/IJDVL_874_2022
An 82-year-old Chinese woman with a history of cataract in both eyes developed an asymptomatic nodulo-plaque on her right cheek 2 years prior to presenting at our hospital. In the last 2 months before admission, the plaque rapidly grew to a huge lump and hindered mastication. There was no evidence of malignancy, any inherited immune deficiency, acquired immune deficiency syndrome or any drugs leading to immunosuppression. Physical examination revealed that the patient had Fitzpatrick skin type III to IV with a 10 × 10 cm firm subcutaneous lesion on the right cheek with a visible reddish-brown nodulo-plaque in the centre and right eyelid ptosis [Figures 1a and 1b]. There were no palpable enlarged lymph nodes. Both chest and abdominal computed tomography (CT) were normal. A punch biopsy was performed that revealed tumour composed of small, uniform, round blue cells arranged in anastomosing cords, bands and clusters in the entire dermis. The cells possessed ill-defined, scanty cytoplasm and round vesicular nuclei with “salt and pepper” chromatin [Figures 2a and 2b]. The immunostaining showed a paranuclear dot-like reactivity for CK20. The NSE, Syn and CgA levels were positive, and TTF-1 was negative [Figure 3].
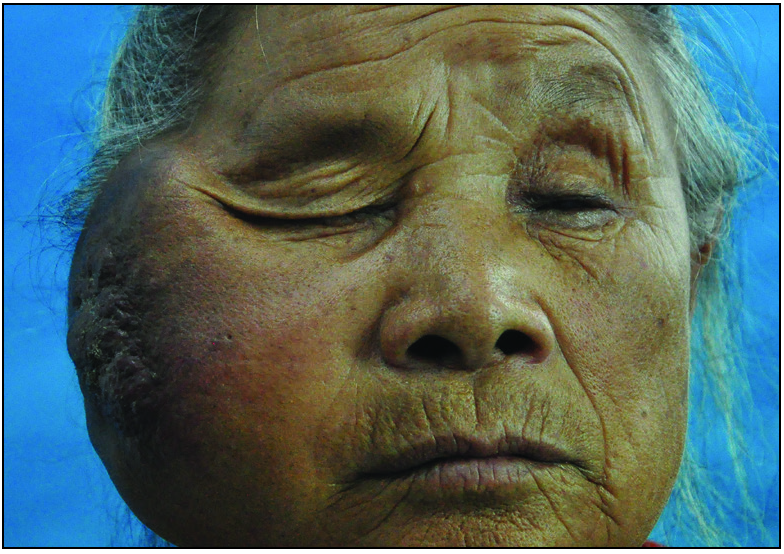
- A huge lump with reddish-brown nodulo-plaque on the right cheek.
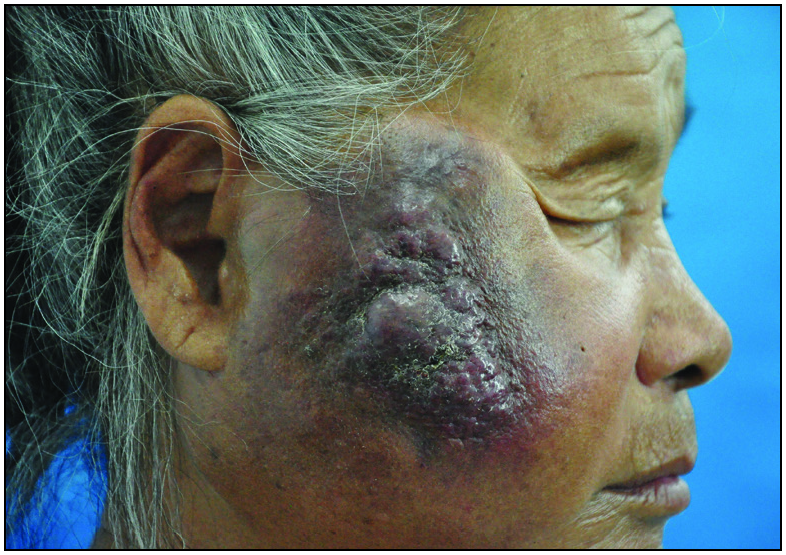
- A huge lump with reddish-brown nodulo-plaque on the right cheek.
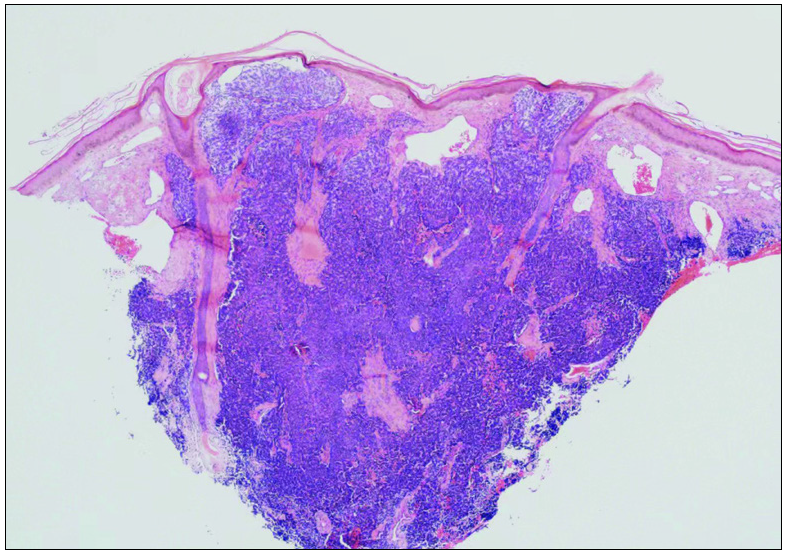
- Tumors composed of small uniform round blue cells arranged in anastomosing cords, bands and clusters in the entire dermis (H&E 40x).
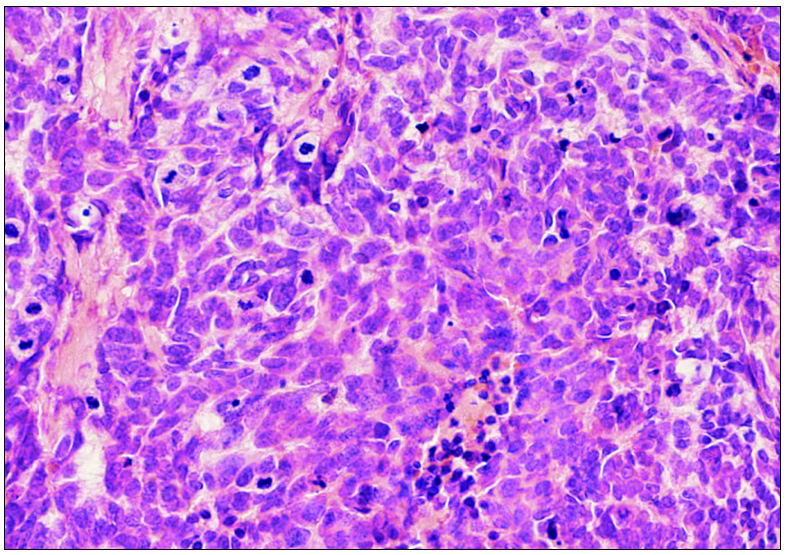
- Cells showing scanty cytoplasm and round vesicular nuclei with “salt and pepper” chromatin (H&E 400x).
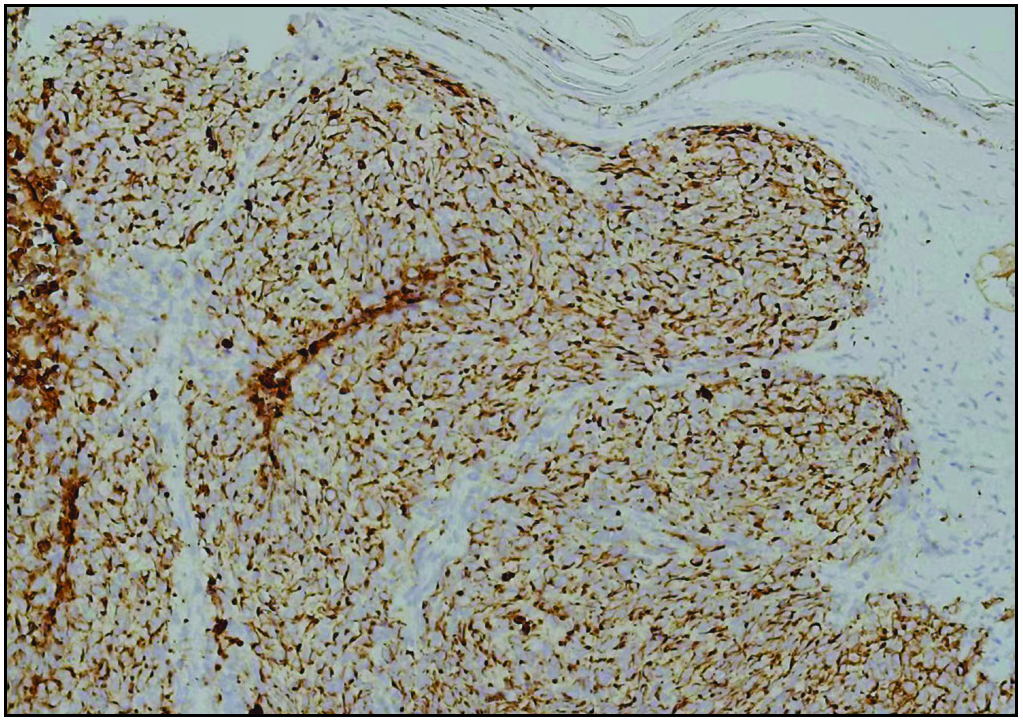
- Paranuclear dot-like CK20 staining (IHC,200x).
Question
What is your diagnosis?
Answer
The patient was diagnosed with Merkel cell carcinoma.
Discussion
Merkel cell carcinoma (MCC) is a rare and aggressive cutaneous malignancy of neuroendocrine origin associated with a high risk of local recurrence and distant metastases. It is typically characterised as a rapidly growing, solitary, asymptomatic lesion. The clinical appearance is often as red–violet, firm, dome-shaped nodules, while sometimes can be pleomorphic, plaque-like, or subcutaneous, flesh-coloured nodules. The disease preferentially affects fair skinned elderly individuals over the age of 70 years with a predilection for sun-exposed sites. Immunosuppression is a known predisposing factor.1 Recently, polyomavirus has been implicated as an aetiological agent in up to 80% of the cases.1
The gold standard for the diagnosis of MCC is skin biopsy and confirmation by histology and immunohistochemistry.2 The tumour is composed of uniform, small, round blue cells with large lobulated nuclei and scanty cytoplasm; mitoses and numerous apoptotic bodies. The cells are present as sheets and solid nests, infiltrating the entire dermis and sometimes extending into the subcutaneous tissue. The histopathologic differential diagnoses include metastatic small cell carcinoma of the lung, small cell cutaneous lymphoma, melanoma, Ewing’s sarcoma and rhabdomyosarcoma. Immunohistochemistry is often used to confirm the diagnosis of MCC. Expression of cytokeratin-20 (CK20) in a paranuclear dot-like pattern is a characteristic feature of the tumour, and positive neuroendocrine markers such as neuron-specific enolase (NSE), synaptophysin (Syn) and chromogranin A (CgA) are also characteristics of MCC on immunohistochemistry analysis. Positive thyroid transcription factor-1 (TTF-1) staining is useful to differentiate small cell carcinoma of the lung from MCC.1 Moreover, the presence of polyomavirus in MCC was positive in both PCR-based detection in 10–15 patients (88–100%) and IHC detection in 10–11 patients (58–65%). It was found that the detection of polyomavirus in the neoplasm helps to distinguish it from other tumours.3 In our case, the immunostaining showed that CK20, NSE, Syn and CgA were positive and TTF-1 was negative, which confirmed the diagnosis of MCC.
MCC has a poor prognosis and high rates of local recurrence and nodal metastasis, which highlights the aggressive nature of this tumour and poses challenges in the treatment and management. The main treatments are surgery and radiotherapy. Surgical resection of the tumour is the first-line therapy, while radiotherapy is the only treatment of choice for inoperable tumours. Previously, chemotherapy was the only alternative in advanced-stage or refractory MCC until several clinical trials1 demonstrated the efficacy of immune-checkpoint inhibitors. Immunotherapy has shown encouraging results in this condition. However, the patient declined further treatment due to financial reasons and poor prognosis. Unfortunately, the tumour eventually grew so large that it compressed the neighbouring tissues leading to dysphagia, and the patient consequently died 3 months later.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline—Update 2022. Eur J Cancer. 2022;171:203-31.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical relevance of liquid biopsy in melanoma and Merkel cell carcinoma. Cancers (Basel). 2020;12:960.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The spectrum of Merkel cell polyomavirus expression in Merkel cell carcinoma, in a variety of cutaneous neoplasms and in neuroendocrine carcinomas from different anatomical sites. Hum Pathol. 2012;43:557-66.
- [CrossRef] [PubMed] [Google Scholar]





