Translate this page into:
A newborn with an oral mass: Non-neural granular cell tumor
Correspondence Address:
Muzeyyen Muge Savas
Department of Pathology, Umraniye Training and Research Hospital, University of Health Sciences, Istanbul
Turkey
| How to cite this article: Savas MM, Zemheri IE. A newborn with an oral mass: Non-neural granular cell tumor. Indian J Dermatol Venereol Leprol 2020;86:57-59 |
Sir,
The granular cell tumor is a common benign mesenchymal neoplasm originating from peripheral neural elements, particularly the Schwann cells and hence strongly positive to S100 protein. The tumor is composed of large, oval, or polygonal cells with abundant eosinophilic granular, Periodic acid-Schiff (PAS) positive cytoplasm. It is more commonly seen in adults and women are affected twice as compared to men. More than 50% of cases arise in the head and neck region.[1]
In contrast to the granular cell tumor, the non-neural granular cell tumor is a rare, subsequently identified diagnosis. It has been 28 years since the first report by LeBoit et al. describing the non-neural granular cell tumor consisting of sheets, nests, and fascicles of granular cells exhibiting cytologic atypia and scattered mitoses proliferating in a polypoid pattern in the absence of staining with S100 protein. Despite the electron microscopy revealing the granules to be lysosomes, immunohistochemical panel demonstrated a lack of differentiation towards any specific structures. The authors, therefore, suggested labelling the tumor primitive.[2] The importance in its histological appearance and lack of differentiation may suggest a low-grade mesenchymal malignancy. In which case, misdiagnosis carries the risk of aggressive treatment for what is essentially a benign tumor.
Till date, several cutaneous and only five oral cases of S100 negative granular cell tumors have been published.[3] No cases of non-neural granular cell tumor with oral involvement in newborn has been reported so far. Here, we describe the youngest case (newborn) with oral S100 negative non-neural granular cell tumor.
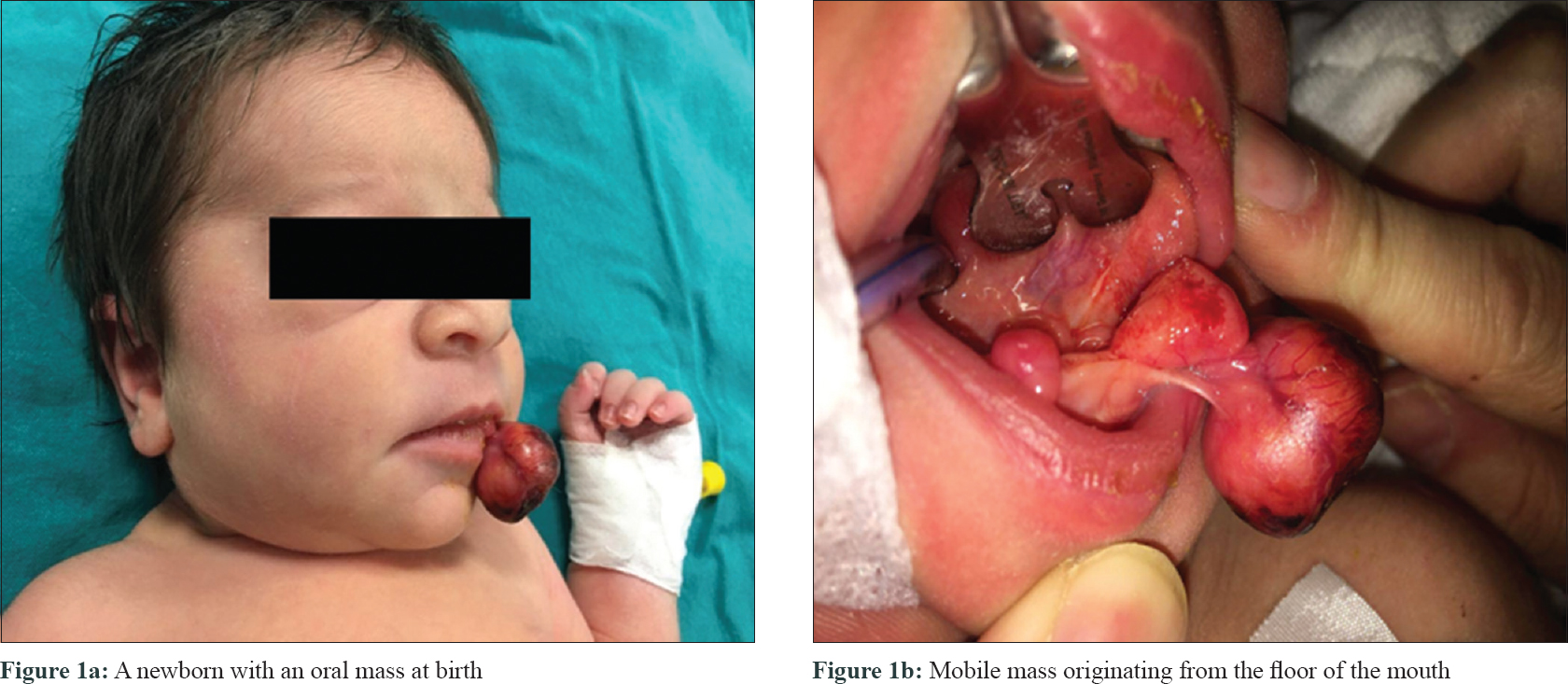
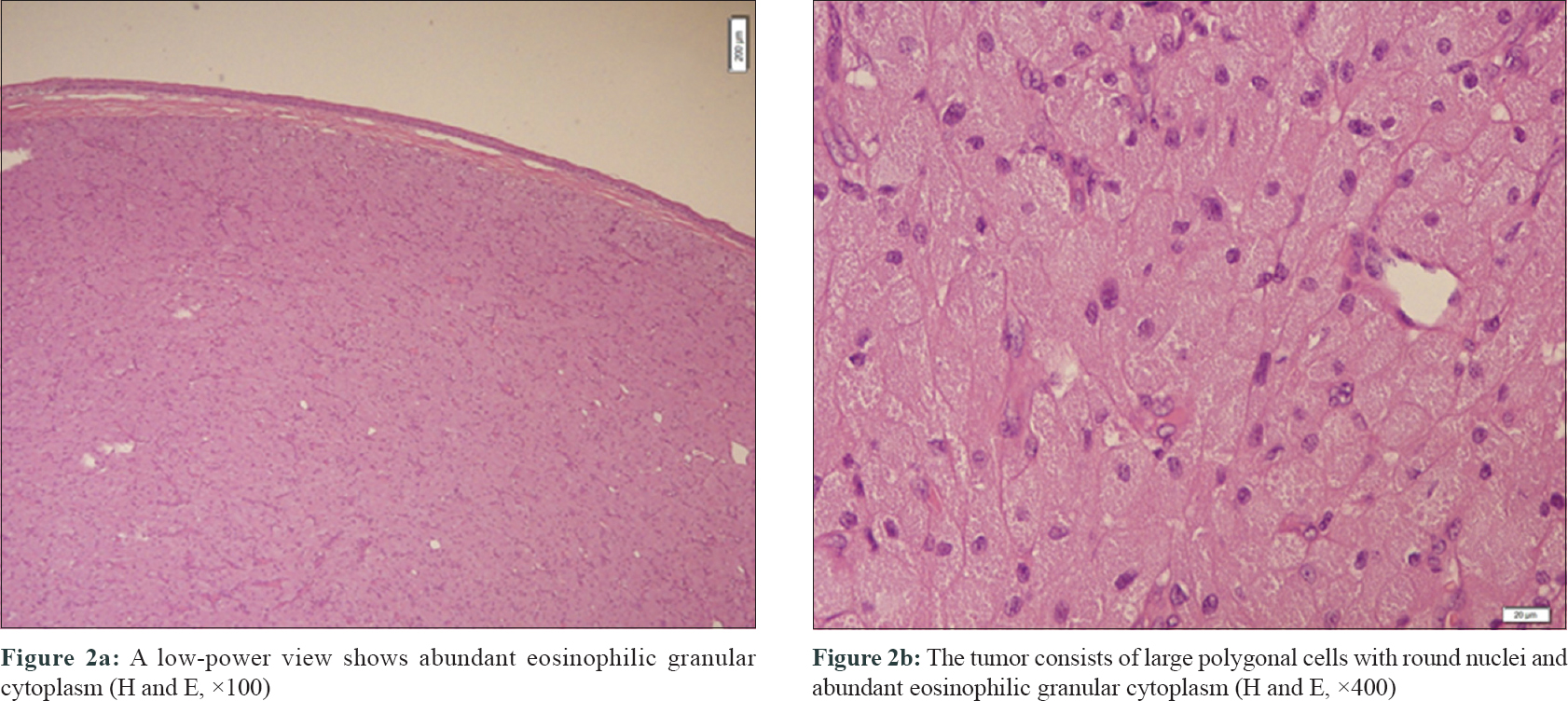
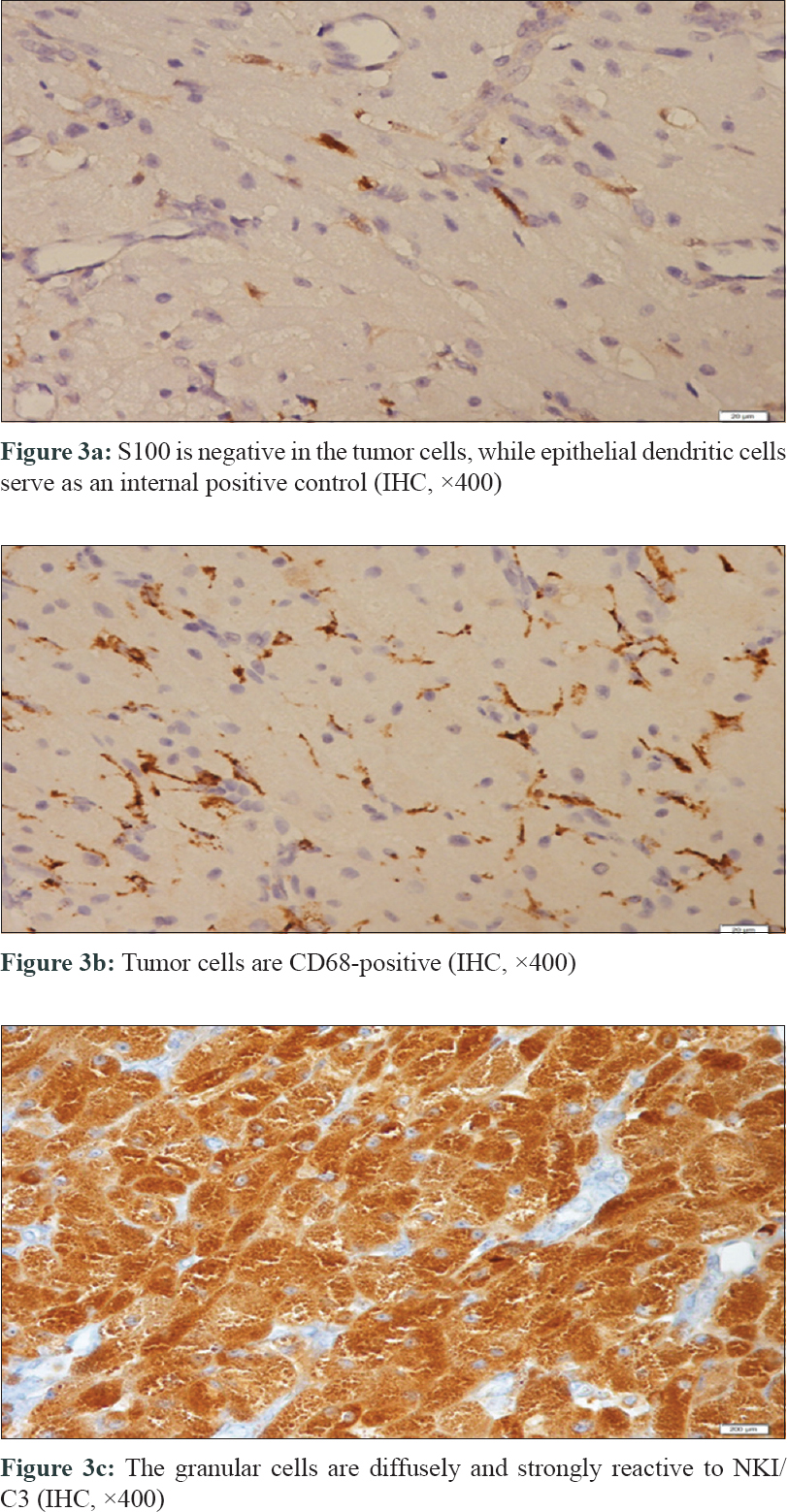
A newborn presented with an oral mass at birth. She was born at 39 weeks through spontaneous vaginal delivery after an uncomplicated pregnancy to a 33-year-old mother who had previous three pregnancies. resulting in a normal child with no significant medical history. The results of the maternal prenatal laboratory tests were all unremarkable. She had received regular prenatal care, and routine ultrasonography revealed no anomalies. Cutaneous examination revealed a 2.2 × 2 × 1.7 cm mobile mass originating from the floor of the mouth and hanging out of the lips [Figure - 1]a, [Figure - 1]b. The mass was firm and round with a “partially granular and lobulated-appearing mucosal surface”. Histopathological examination showed ulcerated fibrovascular connective tissue that contained large polygonal cells with round, euchromatic nuclei and abundant eosinophilic granular cytoplasm. The nuclei were prominent and vesicular, and some cells showed pink nucleoli [Figure - 2]a, [Figure - 2]b. Pleomorphism was not observed and mitoses were rare; approximately one mitosis per 10 high-power fields was observed. Acute and chronic inflammatory cells were noted throughout the lesion with focal germinal center–like aggregates in the deeper connective tissue. Immunohistochemical studies revealed positive staining for neuron-specific enolase, CD63 (NKI/C3), and CD68; but negative for S100 and desmin [Figure - 3]a, [Figure - 3]b, [Figure - 3]c.
 |
| Figure 1: |
 |
| Figure 2: |
 |
| Figure 3: |
The differential diagnosis of oral S100 negative polypoid granular cell tumor includes polypoid lesions of the oral cavity. The congenital granular cell epulis is a rare tumor which is apparent at birth and spontaneously regress thereafter. It affects the maxillary alveolar ridge and consists of S100negative granular cells.[1] On the other hand oral leiomyomas are painful, well-circumscribed submucosal nodule. Histologically conventional findings along with granular cell changes can be seen. This lesion is positive for desmin, smooth muscle actin (SMA), and caldesmon stains. The verruciform xanthoma consists of xanthoma or foam cells. The majority are seen on gingiva and hard palate. The xanthoma cells are CD68, NKI/C3, and CD163 positive and S100 negative. Melanomas and benign melanocytic lesions may also show granular cell changes, which are stained with positive NKI/C3, S100 protein, HMB45 and Melan A.[4]
In the present case, we detected an exophytic, polypoid neoplasm originating from the floor of the mouth in a newborn. Histopathologically we have seen a tumor consisting of sheets, nests, and fascicles of polygonal, oval, or plump spindle cells with copious eosinophilic, granular cytoplasm with prominent vesicular nuclei. The tumor cells were S100 protein, desmin, and HMB45negative however CD68, and NKI/C3 (CD63) were diffusely and strongly positive. We report this case for its rare presentation of a S100 negative non-granular cell tumor of the oral cavity in a newborn.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
| 1. |
Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization Classification of Tumors: Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. p. 163-208.
[Google Scholar]
|
| 2. |
LeBoit PE, Barr RJ, Burall S, Metcalf JS, Yen TS, Wick MR. Primitive polypoid granular-cell tumor and other cutaneous granular-cell neoplasms of apparent nonneural origin. Am J Surg Pathol 1991;15:48-58.
[Google Scholar]
|
| 3. |
Rawal YB, Dodson TB. S-100 negative granular cell tumor (so-called primitive polypoid non-neural granular cell tumor) of the oral cavity. Head Neck Pathol 2017;11:404-12.
[Google Scholar]
|
| 4. |
Patterson JW, editor. Granular cell tumors. In: Weedon's Skin Pathology. 4th ed. Virginia, USA: Elsevier; 2016. p. 1056-9.
[Google Scholar]
|
Fulltext Views
5,183
PDF downloads
3,842





