Translate this page into:
A randomized controlled pilot study of a proprietary combination versus sunscreen in melasma maintenance
Corresponding author: Dr. Gopalsing Rameshsing Rajput, Department of Dermatology, Venereology and Leprology, Institute of Naval Medicine, INHS Asvini, Colaba, Mumbai - 400 005, Maharashtra, India. gopal.270985@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chatterjee M, Neema S, Rajput GR. A randomized controlled pilot study of a proprietary combination versus sunscreen in melasma maintenance. Indian J Dermatol Venereol Leprol 2022;88:51-8.
Abstract
Background:
Melasma is the commonest cause of facial hypermelanosis in skin type IV-VI. First-line treatment includes a triple combination containing topical corticosteroid and hydroquinone which have side effects on prolonged use. Chemical peels are a second-line management option with the laser being used in refractory cases, but the worsening of hyperpigmentation in darker skin types can occur following laser therapy. Sunscreen is a must to prevent relapses.
Aims and Objectives:
(i) To compare the effects of treatment with a proprietary combination (phenyl ethyl resorcinol, nonapeptide-1, aminoethyl phosphinic acid, antioxidants and sunscreen) versus sunscreen alone in limiting or reducing, melasma and preventing recurrence as a maintenance regimen after the initial use of triple combination,(ii) to evaluate the safety of the formulation studied, and (iii) to study the improvement of the quality of life of the patients after using the study formulation versus placebo.
Methods:
It was a prospective double-blinded parallel-group randomized controlled pilot study. A total of 46 subjects were recruited by consecutive sampling methods and randomized to 23 each in case and control groups. The study period was eight months with three phases. Phase 1 constituted the application of triple combination for eight weeks by both groups followed by phase 2 with the case group applying proprietary medicine and the control group applying sunscreen. Phase 3 was a follow-up period to see the sustenance of results in both groups as well as any evidence of relapses. Sunscreen was applied in all three phases.
Results:
Case group in the study showed improvement in the melasma severity score and mean melanin index as measured by mexameter but it did not attain statistical significance as compared to the control group. The melasma area and severity index score showed a consistent reduction in the case group, whereas it increased in the control group from baseline.
Limitations:
Small sample size and a short follow-up period of our study were major limitations.
Conclusion:
The proprietary combination, which has sunscreen as one of its constituents, is more effective in maintaining remission after triple combination without any added inconvenience of application of two separate preparations as compared to sunscreen alone.
Keywords
Hypermelanosis
melasma
sunscreen
Introduction
Melasma is a common cause of facial hyperpigmentation. It presents as a symmetric hypermelanosis of sun-exposed skin characterized by brown macules and patches, usually involving the face in women.1,2 There are three clinical patterns of melasma: centrofacial (most common), malar and mandibular. While the exact cause of melasma is unknown, both genetic and environmental factors are thought to play a role in the development of this condition. Environmental factors include ultraviolet radiation exposure, pregnancy, oral contraceptives, oestrogen-progesterone therapies, thyroid dysfunction, cosmetics and medications.1-3
Melasma is common among women with a darker complexion (Fitzpatrick’s skin type IV-VI).4 Although the prevalence of melasma has not been investigated in most countries, melasma accounts for about 4–10% of new cases in dermatology clinics.5,6 Melasma is a common pigmentation disorder among Indians.7Malar pattern melasma is predominant in the southern region of India to a greater extent when compared with the northern region.8-10
Sun-exposure, pregnancy, use of oral contraceptives and use of cosmetics at least five times a week contribute to the exacerbation of melasma among the Indian population.9
The effect of a socioeconomic class of the patient, effect of previous treatments, menopause status and other concomitant conditions have not been evaluated in epidemiological studies conducted to evaluate the prevalence of melasma.11-13
Several methods of treatment are available to patients with melasma. First-line therapy usually consists of topical compounds that affect the melanin production pathway, broad-spectrum photoprotection and camouflage. Second-line therapy often consists of the addition of chemical peels, although these must be used cautiously in patients with darker skin. Laser and light therapies represent potentially promising options for patients who are refractory to other modalities but also carry a significant risk of worsening the disease. A thorough understanding of the risks and benefits of various therapeutic options is crucial in selecting the best treatment.14 While no single therapy has proven to be of benefit, combinations of modalities can be used to optimize management in difficult cases.14Treatment requires reconsideration as affected patients have melasma for many years and a significant effect on the quality of life has been documented.1,12,15 Because melasma may be present for many years and relapse after improvement is common, the development of a maintenance regimen after initial improvement would help in the management of this disorder.15Such newer treatment modalities on the horizon are a cause of optimism in the management of this chronic disorder with the aim that results may be achieved without steroids and development of a safe maintenance regimen would be possible.14
The present study was a double-blind parallel-group randomized controlled pilot study and it aimed (i) to compare the effects of treatment of a proprietary combination (phenyl ethyl resorcinol, nonapeptide -1, aminoethyl phosphinic acid, antioxidants and sunscreen) vs sunscreen alone (placebo) to limit or reduce melasma and prevent recurrence as a maintenance regimen after the initial use of triple combination (ii) to evaluate the safety of the study formulation and (iii) to study the improvement of the quality of life of the patients after using the study formulation versus placebo.
Methods
This study was conducted in the Department of Dermatology, Command Hospital, Kolkata from November 2015 to September 2016.
Four men and 42 women consecutive patients aged 23–50 years with moderate-to-severe hypermelanosis consistent with a clinical diagnosis of melasma attending the dermatology outpatient clinic in a tertiary care hospital in eastern India were included in this prospective study. Out of 46 patients, 27 were having centrofacial melasma and 19 were diagnosed as having malar melasma. All the patients included in the study were found to have mixed melasma per Wood’s lamp examination.
Inclusion criteria
Patients with a clinical diagnosis of melasma.
Exclusion criteria
History of any treatment for melasma in the last six months; history of any other concomitant facial pigmentary disorders; history of allergy to topical products like sunscreen; history of skin disease which precludes the use of products like topical steroids and some chemical sunscreens, e.g.,rosacea.
The subjects were randomized into two groups by computer-generated software programs: case group and control group. There were three phases of the pilot study.
Phase 1
For the first eight weeks of treatment, both the case group and control group received once a day application of triple combination therapy of tretinoin (0.05%), fluocinolone acetonide (0.01%) and hydroquinone (4%) in the evening for overnight use and sunscreen (SPF 30 with physical blockers)(composition: avobenzone 2 %w/w+octocrylene 3 %w/w+octyl methoxycinnamate 7.5 %w/w+oxybenzone 3 %w/w+zinc oxide 2 %w/w) every 3 hourly starting fromthe morning till 5 PM (last application of day) The total number of visits during phase 1 was 3 (visit 1 - baseline, visit 2 and visit 3) with an interval of 4 weeks ± 3 days. Both groups were advised to apply 3 mL of sunscreen onthe face spreading it evenly to cover the entire area on a specified time interval. Both groups were told to apply triple combination only onthe affected area.
Phase 2
Thereafter, the case group received the study formulation i.e.,phenyl ethyl resorcinol, nonapeptide-1, aminoethyl phosphinic acid, antioxidants andsunscreen for 16 weeks to be applied in the morning and afternoon at 08:00 hours and 14:00 hours and the control group received the placebo i.e.,sunscreen to be applied at the same times. The total number of visits during phase 2 was 4 (visit 4, visit 5, visit 6 and visit 7) with an interval of 4 weeks ± 3 days.
Phase 3
Both the case and control groups had a follow-up phase where the study subjects did not receive any therapy for melasma. The total number of visits during phase 3 was 1 (visit 8) with an interval of 8 weeks ± 3 days.
The demographic and clinical data of the patients including age, gender, age of onset, disease duration, subjective assessment of melasma severity by a physician, melasma area and severity index scoresand melanin content (by use of a Mexameter(R)MX 18 by Courage and Khazaka Electronic, GmbH) were recorded.The most hyperpigmented and the homogenously darkened area was selected for mexameter reading and the site was marked and instructions were given to the patient to remember the site and to indicate the same site for next readings).16 The study was approved by an ethics committee and all participants signed the informed consent.
Statistical analysis
The statistical measures used to analyze the data are described in detail below.
Summary tables (descriptive statistics and/or frequency tables) are provided for all baseline variables, as appropriate. Descriptive statistics were used to summarize the quantitative variables with the number of patients (n), mean, median, standard deviation (SD), minimum and maximum. Frequency and percentages were used to summarize the qualitative/ categorical variables. A paired sample t-test was used to compare two population means in the case of two samples that are correlated. Two sample t-test was used for comparing two treatment groups.
A Chi-square test was used to determine whether there is a significant association between the two variables.
Results
The study included 46 patients with melasma (4 men and 42 women). In the case group, the total number of subjects who completed the study were 17. The primary reason for early termination was lack of follow-up. In the control group, the total number of subjects who completed the study were 14. The primary reason for early termination was again lack of follow-up. Only one subject withdrew consent. Figure 1 gives the trial flow chart according to Consolidated Standards of Reporting Trials (CONSORT). Table 2 shows the demographic information of the subjects.
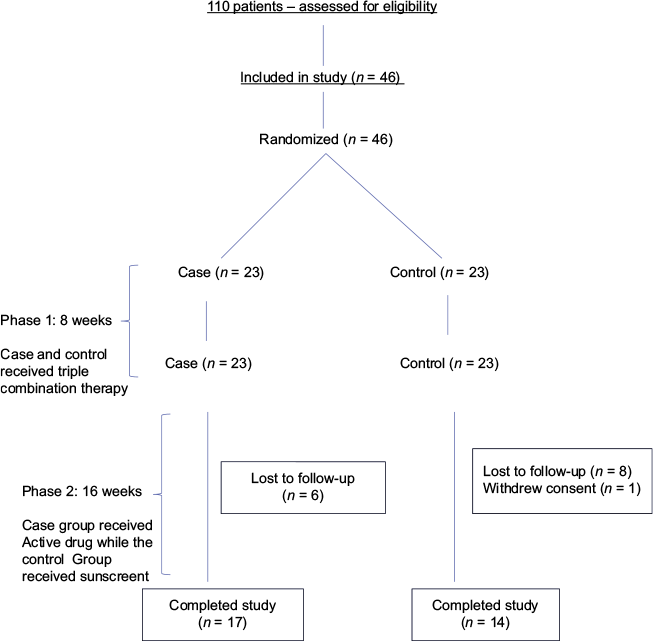
- Study flow chart
| Demographic variable | Case (n=23) | Control (n=23) |
|---|---|---|
| Age (years), mean±SD | 40.05 (7.09) | 37.91 (6.45) |
| Sex, n(%) | ||
| Men | 3 (13.04) | 1 (4.34) |
| Women | 20 (86.95) | 22 (95.65) |
| Changes between visit 3 and visit 7 | ||
|---|---|---|
| Change | Case (n=17) | Control (n=15) |
| Improvement | 1 (5.88) | 0 (0.0) |
| No change | 12 (70.58) | 10 (66.66) |
| Worse | 4 (23.52) | 5 (33.33) |
| P | 0.5456 | |
Phase 1
Figures 2 and 3 represents change in melasma severity during phase 1 when both the groups received triple combination therapy. ‘P’ value at visit 1 between treatment groups is 0.23 and visit 3 is 0.32, suggesting both groups were homogenous at the beginning and end of phase 1.
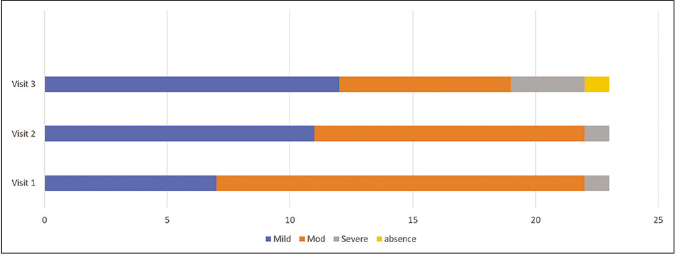
- Change in melasma severity in case group in phase 1
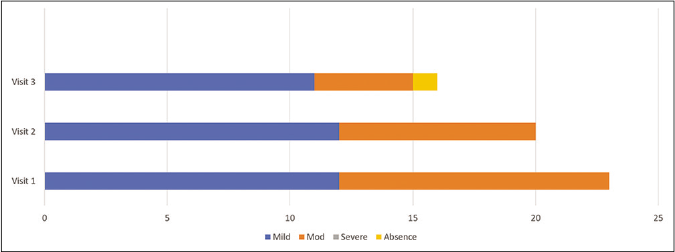
- Change in melasma severity in the control group in phase 1
Mean melanin content in both groups during phase 1 is represented in Figure 4. There was no statistical difference between the case group and the control group at visit 1 (P-value: 0.7223) and visit 3 (P-value: 0.3863). Hence, the data shows the homogeneity between the groups with mean melanin content as measured by mexameter.
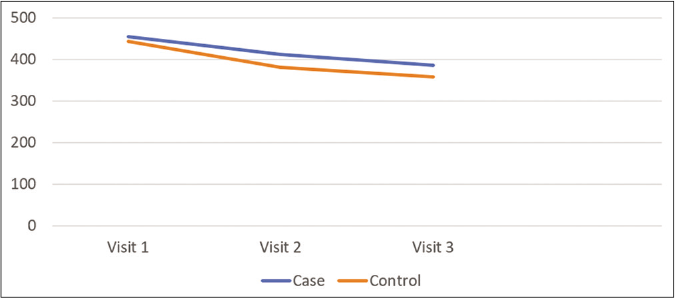
- Change in mean melanin content as assessed by mexameter in phase 1 in case and control group
Phase 2
Table 2 represents the changes in the melasma severity scale between visit 3 and visit 7 where the subjects were receiving either phenyl ethyl resorcinol, nonapeptide -1, aminoethyl phosphinic acid, antioxidants andsunscreen preparation or sunscreen alone. Improvement was more in the case group; however, it didnot reach statistical significance. Figure 5 represents the mean melanin content from visit 3 to 7. Mean melanin content was reduced from visit 3 to visit 7 in the case group more than the control group. Mean melanin content in the case group was 403.9 (±13.25) and in the control group was 412.12 (±30.8) at visit 7. ‘P’ value for difference in melanin content of two groups were 0.59 which did not reach statistical significance.
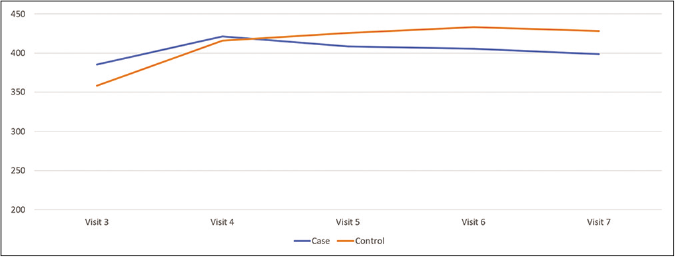
- Change in mean melanin content as assessed by mexameter in phase 2 in case and control group
Phase 3
Table 3 represents the changes in the melasma severity scale between visit 3 and visit 8. After visit 7, both the case and control groups did not receive any treatment for melasma. From the time they received different treatments, 10 (58%) patients showed improvement or no change in melasma severity in the case group, while 7 (42%) patients showed worsening of symptoms. Five (35%) patients showed no change while 9 (65%) patients showed worsening of symptoms in the control group. However, this difference did not reach statistical significance.
| Changes between visit 3 and visit 8 | ||
|---|---|---|
| Change | Case (n=17) | Control (n=14) |
| Improvement | 1 (5.88) | 0 (0.0) |
| No change | 9 (52.94) | 5 (35.71) |
| Worse | 7 (41.17) | 9 (64.28) |
| P | 0.5548 | |
Table 4 shows the change in melasma severity as per the melasma area and severity index score in various phases of treatment.The mean melasma area and severity index score has reduced from 9.9 at baseline to 7.8 at visit 8 in the case group whereas it increased from 9 at baseline to 9.9 at visit 8 in the control group.
| Visit | Phase 1 | |
|---|---|---|
| Case group (n=23) | Control group (n=23) | |
| Visit 1 – Mean (SD) | 9.9 (4.54) | 9.0 (5.30) |
| Visit 3 | 7.3 (4.49) | 5.5 (3.51) |
| Phase 2 | ||
| Visit 4 | 8.2 (4.75) | 8.6 (4.40) |
| Visit 7 | 7.65 (4.41) | 8.5 (4.46) |
| Phase 3 | ||
| Visit 8 | 7.8 (2.93) | 9.9 (4.30) |
Table 5 shows that the adverse effects seen in phase 1 in both groups were similar. Adverse effects in phase 2 were much less in the case group (5.8%) as compared to the control group (26%). Skin itching was complained by one patient in the case group.
| Event | Study Phase 1 | |
|---|---|---|
| Case (n=21), n(%) | Control (n=16), n(%) | |
| Skin burning sensation without any lesion | 1 (4.76) | 1 (6.25) |
| Allergic contact dermatitis | 0 | 1 (6.25) |
| Xerosis | 0 | 1 (6.25) |
| Erythema | 1 (4.76) | 0 |
| Pruritus | 1 (4.76) | 0 |
| Event | Study Phase 2 | |
| Case (n=17), n(%) | Control (n=15), n(%) | |
| Pruritus | 1 (5.88) | 1 (6.66) |
| Allergic contact dermatitis | 0 | 1 (6.66) |
| Skin burning sensation without any lesion | 0 | 1 (6.66) |
| Erythema | 0 | 1 (6.66) |
Table 7 displays the mexameter reading visit wise P value evaluation of mealnin content. Table 8 depicts the evaluation of melanin content. Overall there was no difference in results among men and women.
| Statistical parameter | Group A (n=23) | Group B (n=23) | P# |
|---|---|---|---|
| Visit 1 | |||
| n | 23 | 23 | |
| Missing | 0 | 0 | |
| Mean±SD | 453.9 (110.06) | 442.6 (104.49) | 0.7223 |
| Median | |||
| (minimum–maximum) | 431.3 (272–689) | 419.3 (281–743) | |
| Visit 2 | |||
| n | 23 | 20 | |
| Missing | 0 | 0 | |
| Mean±SD | 411.2 (110.86) | 381.2 (101.12) | 0.3615 |
| Median | |||
| (minimum–maximum) | 402.0 (230–649) | 354.0 (178–590) | |
| Change from Visit 1 | |||
| n | 23 | 20 | |
| Missing | 0 | 3 | |
| Mean±SD | −42.7 (60.49) | −64.6 (80.85) | |
| Median | |||
| (minimum- | |||
| maximum) | −42.7 (−183–111) | −42.2 (−355–14.0) | |
| P* | 0.0027 | 0.0020 | |
| Visit 3 | |||
| n | 21 | 16 | |
| Missing | 0 | 0 | |
| Mean±SD | 385.5 (123.75) | 358.2 (62.14) | 0.3863 |
| Median | |||
| (minimum- | |||
| maximum) | 346.0 (204–665) | 349.2 (225–473) | |
| Change from Visit 1 | |||
| n | 21 | 16 | |
| Missing | 2 | 7 | |
| Mean±SD | −75.7 (81.90) | −74.3 (89.43) | |
| Median | |||
| (minimum- | |||
| maximum) | −56.7 (−273–40.3) | −58.5 (−312–46.3) | |
| P* | 0.0004 | 0.0046 |
#Two sample t-test is used, *Paired t-test is used, change=Visit j –Visit 1; j=2,3. SD: Standard deviation
| Statistical parameter | Group A (n=23) | Group B (n=23) | P |
|---|---|---|---|
| Visit 3 | |||
| n | 21 | 16 | |
| Missing | 0 | 0 | |
| Mean±SD | 385.5 (123.75) | 358.2 (62.14) | 0.3863 |
| Median | |||
| (minimum–maximum) | 346.0 (204–665) | 349.2 (225–473) | |
| Visit 4 | |||
| n | 20 | 15 | |
| Missing | 0 | 0 | |
| Mean±SD | 426.1 (116.16) | 412.0 (79.25) | 0.6884 |
| Median | |||
| (minimum–maximum) | 414.8 (262–673) | 416.8 (283–563) | |
| Change from Visit 3 | |||
| n | 20 | 15 | |
| Missing | 1 | 1 | |
| Mean±SD | 48.1 (76.47) | 52.8 (69.60) | |
| Median | |||
| (minimum-maximum) | 52.4 (−120–231) | 52.7 (−138–158) | |
| P* | 0.0111 | 0.0108 | |
| Visit 5 | |||
| n | 17 | 14 | |
| Missing | 0 | 0 | |
| Mean±SD | 441.1 (98.68) | 425.5 (94.80) | 0.6604 |
| Median | |||
| (minimum–maximum) | 438.2 (315–651) | 401.0 (284–682) | |
| Change from Visit 3 | |||
| n | 17 | 14 | |
| Missing | 4 | 2 | |
| Mean±SD | 73.2 (85.11) | 98.3 (69.82) | |
| Median | |||
| (minimum–maximum) | 65.0 (−55–244) | 85.6 (22.3–288) | |
| P* | 0.0070 | 0.0027 | |
| Visit 6 | |||
| n | 17 | 14 | |
| Missing | 0 | 0 | |
| Mean±SD | 445.2 (93.21) | 432.9 (93.88) | 0.7180 |
| Median | |||
| (minimum–maximum) | 432.7 (318–619) | 413.6 (285–662) | |
| Change from Visit 3 | |||
| n | 17 | 14 | |
| Missing | 4 | 2 | |
| Mean±SD | 73.2 (85.11) | 98.3 (69.82) | |
| Median | |||
| (minimum–maximum) | 65.0 (−55–244) | 85.6 (22.3–288) | |
| P* | 0.0025 | 0.0007 | |
| Visit 7 | |||
| n | 17 | 14 | |
| Missing | 0 | 0 | |
| Mean±SD | 441.6 (101.88) | 435.1 (95.25) | 0.6604 |
| Median | |||
| (minimum–maximum) | 412.0 (269–630) | 401.8 (285–641) | |
| Change from Visit 3 | |||
| n | 17 | 14 | |
| Missing | 4 | 2 | |
| Mean±SD | 73.2 (85.11) | 98.3 (69.82) | |
| Median | |||
| (minimum–maximum) | 65.0 (−55–244) | 85.6 (22.3–288) | |
| P* | 0.0079 | 0.0002 | |
| Visit 8 | |||
| n | 17 | 14 | |
| Missing | 0 | 0 | |
| Mean±SD | 462.5 (94.98) | 455.2 (110.81) | 0.8445 |
| Median | |||
| (minimum–maximum) | 468.3 (307–610) | 420.7 (297–719) | |
| Change from Visit 3 | |||
| n | 17 | 14 | |
| Missing | 4 | 2 | |
| Mean±SD | 73.2 (85.11) | 98.3 (69.82) | |
| Median | |||
| (minimum–maximum) | 65.0 (−55–244) | 85.6 (22.3–288) | |
| P* | 0.0027 | 0.0002 |
#Two sample t-test is used, *Paired t-test is used, change=Visit j –Visit 3; j=4, 5, 6, 7, 8. SD: Standard deviation
Discussion
Melasma is a common acquired disorder of hyper- pigmentation. First-line treatment of melasma includes a triple combination of hydroquinone, tretinoin and fluocinolone. This triple combination is more effective than hydroquinone alone in clearing melasma; however, continuous use may lead to adverse effects like telangiectasia and skin atrophy from the steroid component. Triple combination creams should not be used for more than eight weeks to prevent these adverse effects.17 The alternative approach is to use triple combination intermittently as maintenance therapy.18 The mometasone-based triple combination should be avoided as it leads to steroid-related adverse effects in a large number of patients.19 Broad-spectrum sunscreen should always be prescribed in the management of melasma, both during the clearing and maintenance phase. Physical sunscreen with visible light filters is more effective in the management of melasma.20
Maintenance of remission in melasma after clearing has been achieved has remained a big challenge, as first-line therapy like triple combination can lead to adverse effects if used continuously. Other agents available for long-term maintenance are azelaic acid, kojic acid, arbutin, glycolic acid and topical tranexamic acid. Phenyl ethyl resorcinol, nonapeptide -1, aminoethyl phosphinic acid, antioxidants and sunscreen combination is a new topical for maintenance therapy of melasma. Unlike other drugs that are used for maintenance therapy of melasma, it has sunscreen compounded in it and patients do not need to apply sunscreen separately improving compliance. Besides, irritation potential is much less in this preparation due to absence of such ingredients.
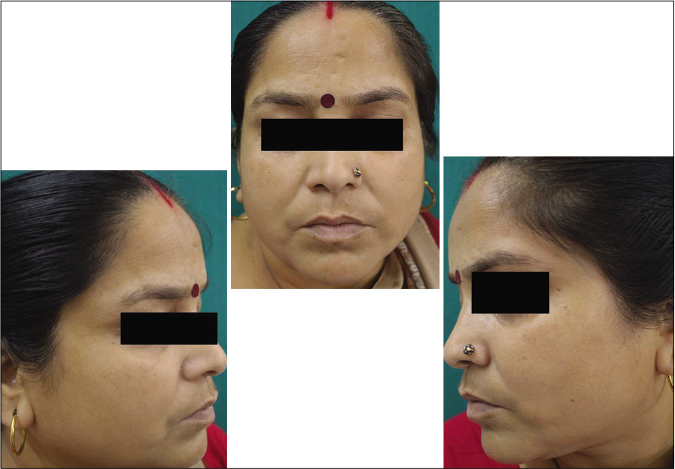
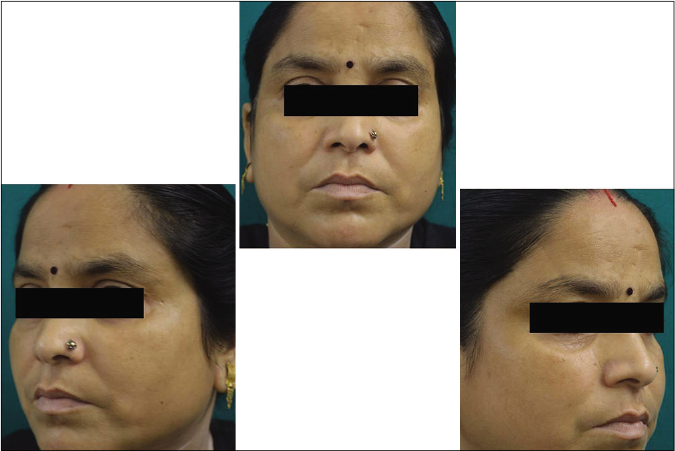
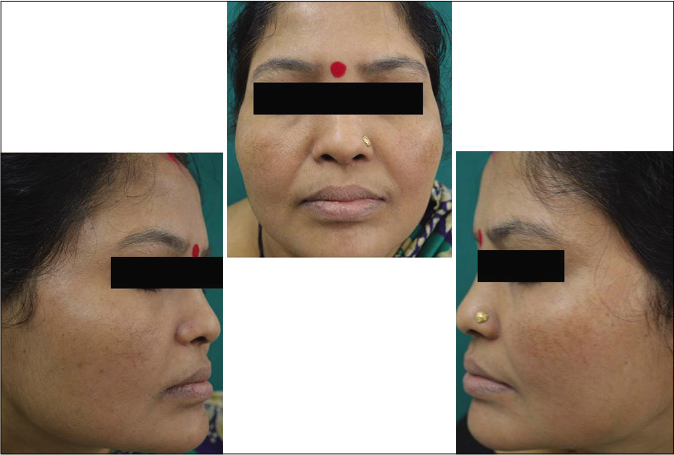
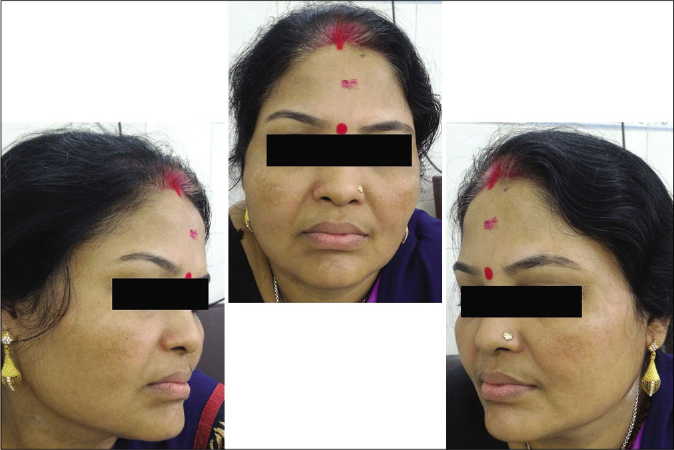
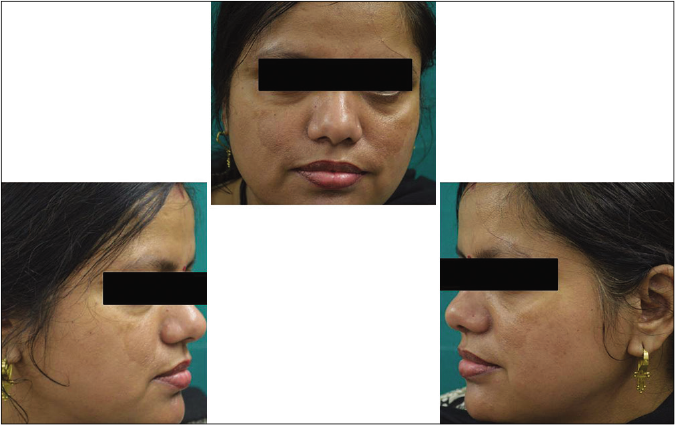
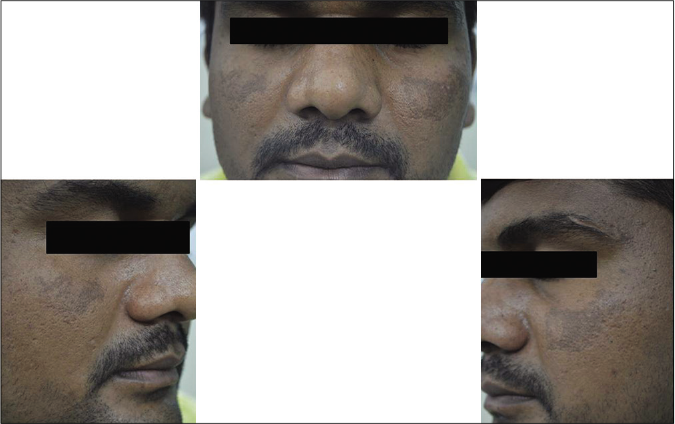
Our study showed that though the mean melasma area and severity index scores at baseline of the cases were higher than that of controls (though not statistically significantly so) and remained so till the end of phase 1, at the end of phase 2 and 3 of the study, the mean melasma area and severity index of the cases were consistently maintained lower than the controls. It also showed that more patients maintained improvement with the combination agent [Figures 6-11] as compared to sunscreen alone at the end of phase 2 and phase 3, [Figures 12-17] as assessed by the melasma severity scale.
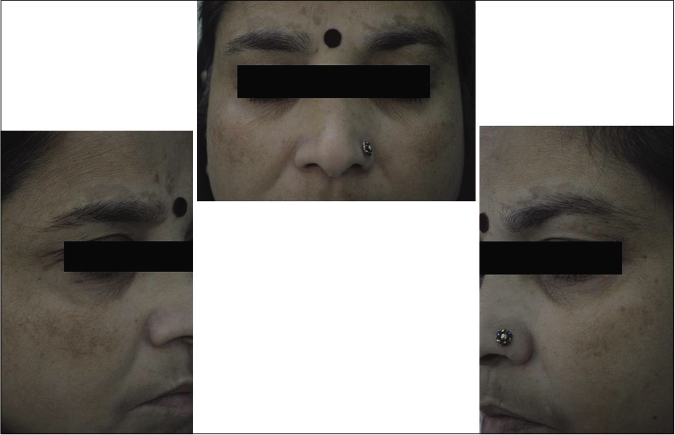
- Case group subject 1 (visit 1)
- Case group subject 1 (visit 3)
- Case group subject 1 (visit 8)
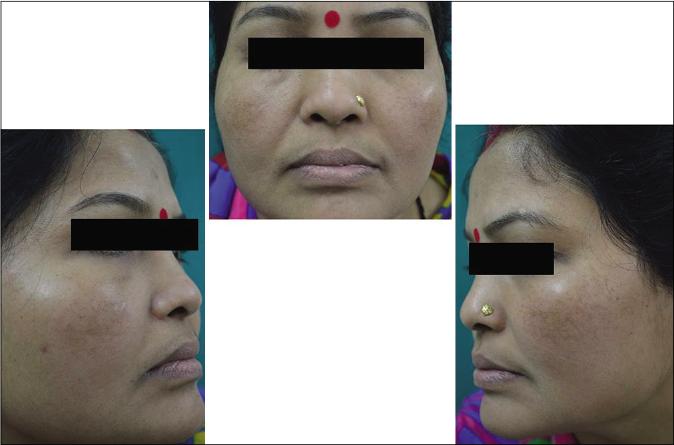
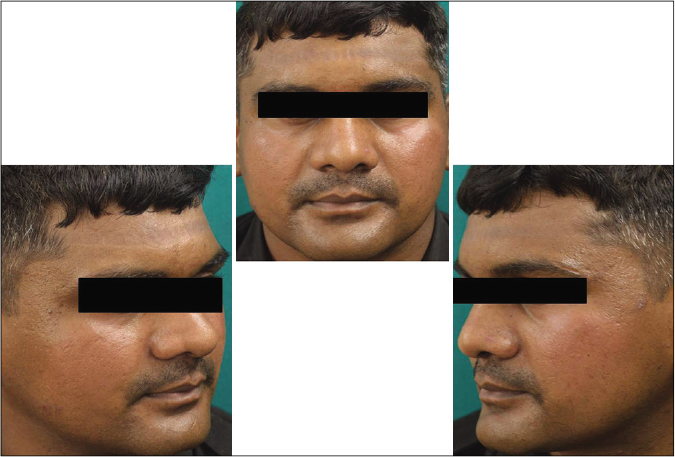
- Case group subject 2 (visit 1)
- Case group subject 2 (visit 3)
- Case group 2 (visit 8)
- Control group subject 1 (visit 1)
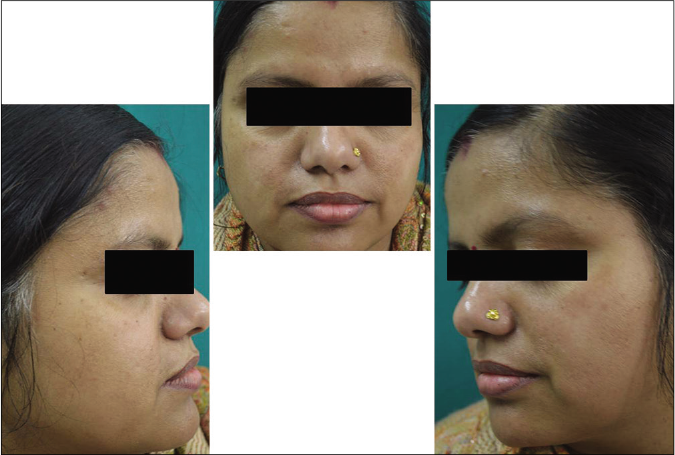
- Control group subject 1 (visit 3)
- Control group subject 1 (visit 8)
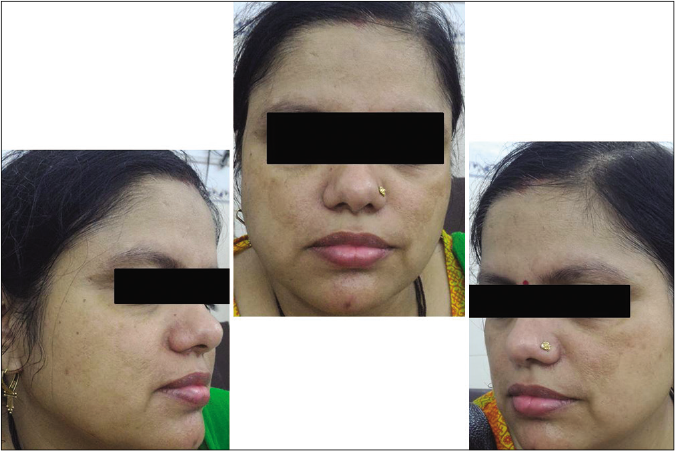
- Control group subject 2 (visit 1)
- Control group subject 2 (visit 3)
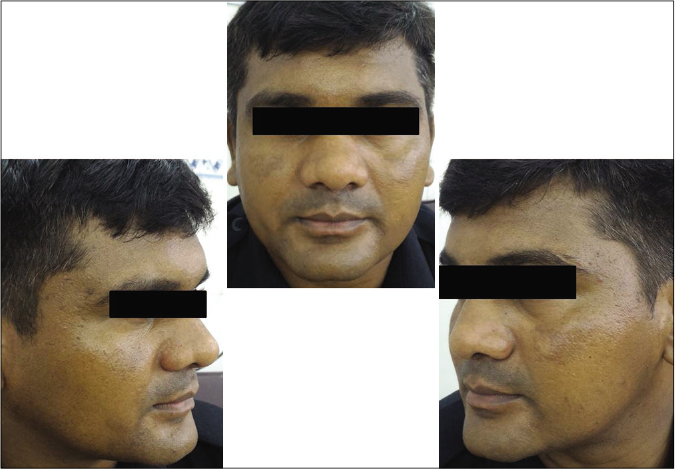
- Control group subject 2 (visit 8)
The stepwise mexameter readings reflected the same findings which elucidate the benefit of the combination which is also associated with overall less adverse effects as compared to sunscreen alone.
Limitations
The major limitations of our study are that it is a pilot study with a small sample size and short follow-up of 16 weeks, which led to results that did not reach statistical significance.
Conclusion
In this pilot study, the proprietary formulation was found to be more effective as compared to the control (sunscreen) arm for maintenance of remission of melasma achieved by triple combination therapy. It also has the added benefit of minimal adverse effects. It can be used for prolonged periods for maintenance of melasma therapy in place of sunscreens with better results as compared to sunscreen alone. It can be used with the same periodicity as sunscreen and reduces the relapse of melasma. Its periodicity of use essentially ensures that melasma remains under better control with no added inconvenience to the patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This study was supported by M/S Palsons Derma, India.
Conflicts of interest
The study has been supported by M/S Palsons Derma. However, the results have been assessed independently and analyzed without any support.
References
- Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-7.
- [CrossRef] [Google Scholar]
- Melasma Etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-7.
- [CrossRef] [Google Scholar]
- Cutaneous epidemiology in two sectors of Guerrero, Mexico. Dermatologia Rev Mex. 1992;36:29-34.
- [Google Scholar]
- Incidence of skin disease in Cuzco, Peru. Int J Dermatol. 1992;31:560-1.
- [CrossRef] [Google Scholar]
- Melasma: A clinicoepidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-2.
- [CrossRef] [Google Scholar]
- Melasma: A clinicopathological study of 43 cases. Indian J Pathol Microbiol. 2009;52:357-9.
- [CrossRef] [Google Scholar]
- Melasma (chloasma): A review with current treatment options. Indian J Dermatol. 2004;49:165-76.
- [Google Scholar]
- Melasma and its association with different types of nevi in women: A case-control study. BMC Dermatol. 2008;8:3.
- [CrossRef] [Google Scholar]
- Melasma: Measure of the impact on quality of life using the French version of MELASQOL after cross-cultural adaptation. Acta Derm Venereol. 2010;90:331-2.
- [CrossRef] [Google Scholar]
- The prevalence of melasma and its association with quality of life in adult male Latino migrant workers. Int J Dermatol. 2009;48:22-6.
- [CrossRef] [Google Scholar]
- Melasma: A comprehensive update II. J Am Acad Dermatol. 2011;65:699-714.
- [CrossRef] [Google Scholar]
- Continuous therapy followed by a maintenance therapy regimen with a triple combination cream for melasma. J Am Acad Dermatol. 2010;62:962-7.
- [CrossRef] [Google Scholar]
- Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64:78-83, 83.e1-2
- [CrossRef] [Google Scholar]
- A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6:36-9.
- [CrossRef] [Google Scholar]
- Preventing melasma recurrence: Prescribing a maintenance regimen with an effective triple combination cream based on long-standing clinical severity. J Eur Acad Dermatol Venereol. 2012;26:611-8.
- [CrossRef] [Google Scholar]
- Mometasone-based triple combination therapy in melasma: Is it really safe? Indian J Dermatol. 2010;55:359-62.
- [CrossRef] [Google Scholar]
- Near-visible light and UV photoprotection in the treatment of melasma: A double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- [CrossRef] [Google Scholar]






