Translate this page into:
A randomized study to evaluate the efficacy and effectiveness of two sunscreen formulations on Indian skin types IV and V with pigmentation irregularities
2 CIDP Biotech India Pvt. Ltd., New Delhi, India
3 L'Oréal Research and Innovation, Mumbai, Maharashtra, India
4 L'Oréal Research and Innovation Campus, Clichy, France
Correspondence Address:
Rashmi Sarkar
Department of Dermatology and Venereology, Ward No. 22/23 (Through Gate No. 3), Maulana Azad Medical College and Lok Nayak Hospital, Bahadur Shah Zafar Marg, New Delhi - 110 002
India
| How to cite this article: Sarkar R, Garg VK, Jain A, Agarwal D, Wagle A, Flament F, Verschoore M. A randomized study to evaluate the efficacy and effectiveness of two sunscreen formulations on Indian skin types IV and V with pigmentation irregularities. Indian J Dermatol Venereol Leprol 2019;85:160-168 |
Abstract
Background: Regular exposure to ultraviolet rays is high in India, where most Indians present Fitzpatrick skin phototypes IV and V.
Aims: To evaluate the efficacy and compare the effectiveness of two sunscreen products on Indian skin types IV and V with pigmentation irregularities.
Methods: A randomized, uncontrolled and investigator-blinded, single-center study enrolled adult men and women (18–45 years) with Fitzpatrick skin phototypes IV (28° < individual typological angle <10°) and V (10° < individual typological angle < −30°) with pigmentary abnormalities seen on the face in adults (actinic lentigines and postinflammatory hyperpigmentation), who did not use sunscreens. Participants were randomized (1:1) to either of the two marketed sunscreen products, Product A (sun protection factor 50 PA+++) or Product B (sun protection factor 19 PA+++), applied twice daily before sun exposure for ≥2 h. Primary objectives aimed at assessing possible improvement in hyperpigmented spots and overall skin appearance after 12 weeks of use. Evaluation of skin radiance and skin color was done by means of L'Oréal color chart and colorimetric measurements (Chromameter®).
Results: Among the 230 enrolled participants, 216 (93.91%) completed the study. The clinical assessment of the density of pigmented spots and skin radiance showed significant (P < 0.001) improvement in both groups during all visits. The qualitative (participant perception) and quantitative (Chromameter®) data indicated improvement in pigmentation from Week 0 to Week 12. Both products were well-tolerated.
Limitations: The study was conducted over a rather short period of time (12 weeks) at a single location.
Conclusions: This is the first study conducted on Indian skin phototypes IV and V under real-life conditions. It demonstrated the effect of regular sunscreen usage in the prevention of certain signs of skin photoaging such as increased pigmentation or pigmentary abnormalities, thus providing support and assistance to clinicians in suggesting the use of efficient sun-screening products to patients.
Introduction
India is geographically located in Asia; however, describing Indian skin as Asian skin or skin of color may not be accurate. Indian skin color is diverse with Fitzpatrick phototypes varying from III in North India to VI in South India, with the majority of population having phototypes IV (28° < individual typological angle <10°) and V (10° < individual typological angle < −30°). The latitude and environmental conditions have a great impact on the Indian skin color.[1],[2],[3],[4] With regard to the geographical location and the vast territory of India, ultraviolet radiance (Ultraviolet B: 290–320 nm; Ultraviolet A: 320–400 nm) shows a wide mosaic of variations, reaching very high intensities in some southern Indian regions. Both Ultraviolet B and Ultraviolet A rays induce skin changes depending upon the skin compartment, dermis or epidermis. Ultraviolet A penetrates deep into the skin and greatly affects the dermal connective tissue. The melanin content in the darker skin types offers some protection against ultraviolet rays, as compared to lighter skin tones.[5],[6]
Previous studies, using the Episkin® reconstructed skin model, have demonstrated the biological impact of Ultraviolet A on the whole skin, which includes oxidative stress, increased pigmentation and modulation of gene expression.[7] Increased pigmentation by Ultraviolet A is mainly due to photooxidation of preexisting melanin or melanin precursors and is therefore more pronounced in dark skins, as compared to lighter skin.[8] Ultraviolet B rays can damage the DNA of epidermal cells and induce sunburn reaction, which may result in photocarcinogenesis with long-term exposure.[9] Overall, ultraviolet rays are responsible for the onset or exacerbation of melasma, postinflammatory hyperpigmentation, photodermatoses, photoaging and actinic lentigines, with melanosomes showing variability in size and density, especially in dark skins.[10],[11] Following ultraviolet exposure, reorganization of melanosomes in the skin upper layers is more pronounced in dark skins. These pigmentary disorders are a major concern in Indians and have a great psychosocial impact on their quality of life. Photoprotective products with a well-balanced Ultraviolet B/Ultraviolet A protection are most efficient against daily ultraviolet-induced pigmentation.[12],[13],[14],[15],[16],[17]
Most in vivo photoprotective studies are conducted with Fitzpatrick skin types I, II and III (lighter skins) and show improvements in skin aging and texture, whereas data on skin types IV and V are scarce. This is the first in vivo real-life study conducted in New Delhi under Indian environmental conditions to evaluate the effect of two marketed sunscreen products on skin types IV and V with pigmentation irregularities. The primary objective was to assess the improvement over baseline in pigmentation irregularities, and the overall skin appearance after 12 weeks of daily sunscreen use and to compare product efficacy between the two sunscreen formulations.
Methods
Study population
Overall, 58 healthy men and 172 women (age: 18–45 years) with pigmentation abnormalities normally seen on the face in adults which included actinic lentigines and postinflammatory hyperpigmentation[18] and with Fitzpatrick skin phototypes IV and V were evaluated from June 1, 2015 to October 9, 2015 at Maulana Azad Medical College, New Delhi, India. New Delhi is located at 28.6°N latitude and 77.2°E longitude, and is situated 222 m above sea level. The average ultraviolet index during this period ranged from 7 to 9 (World Health Organization classification). The study included participants who did not use sunscreens, and who agreed to daily apply a photoprotective product before being exposed to sunlight for 2 hours between 12 PM and 3 PM, but not for more than 4 hours. Women who were free from menopause and with a stable hormonal status were included in the study. Furthermore, men or women who had started, stopped or changed hormonal treatment(s) in the previous one month prior to the study were not included. The study included some male participants who had occupational sun exposure as they were sales representatives, and women who had exposure as per their regular habits, in addition to the study requirements during peak hours.
Key exclusion criteria were the following: Any significant skin pathology on the test areas; hypersensitivity to study products or constituents; any topical/systemic/surgical/physical treatment(s) on the test areas (laser, peel, dermabrasion, etc.) 4 weeks prior to and/or planned; any herbal and/or cosmetic treatments (facials, massage, face packs, etc., [including homemade]) 2 weeks prior to and/or planned; any report of or plan to sunbathe or overexpose to ultraviolet light (mountains sports, phototherapy, tanning salon use, etc.) the month prior to and/or during the study.
The protocol was approved by the Institutional Ethics Committee at the study site. The study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, applicable regulatory requirements, and in compliance with the study protocol. All participants provided written and audiovisual informed consents to participate.
Study design and treatments
This investigator-blinded, parallel group, single-center study consisted of a 2-week screening and wash-out period, and a 12-week treatment period (visit once every 4 weeks). The study was conducted during the summer season from June to October and the ultraviolet index ranged 7–9 during this period. Participants were randomized (1:1 [women: men = 3:1]) to either Product A (Vichy Capital Soleil Dry Touch® sun protection factor 50 PA+++) or Product B (Garnier White Complete® sun protection factor 19 PA+++). Participants were instructed to apply the sunscreen product (2 mg/cm2) twice daily (≥3 h interval in-between applications) on their whole face, and expose to sunlight (20–30 min post each application) for ≥2 h; not to have ultraviolet-light sessions, use any topical/cosmetic product unless approved by the investigator, undergo physical treatment on the investigational areas and not to use any fairness product. The use of their individual routine skin cleansing products was allowed.
To ensure investigator blinding, both products were reconditioned in similar blind-coded packaging. A randomization list, stratified by gender and comprising randomization numbers and product codes, was provided to an unblinded person for distributing the product.
Study objectives
The primary objective was to assess the improvement over baseline in pigmentation irregularities, and the overall skin appearance after 12 weeks of daily sunscreen use and to compare product efficacy. The secondary objectives were: (i) to evaluate improvement in hyperpigmented spots through participant's self-assessment, (ii) to compare the products, (iii) to evaluate improvement in skin radiance, and (iv) to evaluate changes in skin color, using the L'Oréal Color Chart® by the dermatologist and participants.
Dermatological evaluation
Skin examinations were performed at the screening visits, i.e., at baseline (Day 0), Week 4 (Day 28 ± 5), Week 8 (Day 56 ± 5) and Week 12 (Day 84 ± 5) to collect information on hyperpigmented spots, or skin abnormalities, especially local intolerance (cutaneous irritation, sensitization or photosensitivity). A stencil marking was performed to ensure that the same site was captured for measurement at all visits. Participants were provided a diary to record the application of the products at home and review at each visit for checking compliance. All adverse events, when occurring, were recorded including their onset, intensity, duration, etc.; use of concomitant medications was also recorded.
Clinical evaluations
The clinical evaluation for pigmentation was assessed by the density of pigmented spots (i.e., the number of spots per unit area) using visual analog scales as defined by the skin aging Female and Male Atlas.[17] Skin radiance was evaluated by the clinical scale ranging from very radiant (0–1), somewhat radiant (2–3), neither dull nor radiant (4–6), somewhat dull (7–8) to very dull (9–10); i.e., a lower score implying an improvement in radiance.
The dermatologist also evaluated the skin color using the Skin Color Map [Figure - 1], each square is coded by a letter A to G, which corresponds to a shade, and 1–24 which corresponds to the degree of lightness (shade 1 = lightest shade; shade 24 = darkest shade).[19] Assessment was performed under standard light conditions to assess a perceivable shift in skin color.
 |
| Figure 1: L'Oréal color chart (skin color map). A to G corresponds to a shade; 1 to 24 corresponds to the degree of lightness; Shade A1 corresponds to the lightest shade, G24 the darkest shade |
Instrumental evaluation
A zone from the left and right cheeks was chosen 2.5 cm from the nasolabial fold for measurement of change in skin color. At each visit, the change in skin color was evaluated using Minolta CR400 R Chromameter®. The average of three successive measurements of three chromametric parameters L* (black to white), a* (green to red) and b* (blue to yellow) components were calculated, along with the individual typological angle = [arctan ([L* − 50)/b*) × 180/3.14)].[11]
Self-assessment
A self-assessment questionnaire was provided to the participants for recording their perception on product efficacy. Participants graded themselves on a 1–10 scale (1 = no improvement; 10 = extremely improved) on fairness, glow/radiance, skin tone evenness and dark spot reduction. Participants also assessed the shift in skin color, using the L'Oréal color chart tool.
Statistical analyses
Sample size was calculated using the clinical score for the density of pigmented spots as primary efficacy criteria. Using 90% power index and a 5% significance level, a sample size of 100 was found appropriate for a change of ≥0.5 units in the clinical scores across time. Thus, for the two product groups, 200 participants were planned for enrolment. Assuming a 15% dropout, 230 participants were enrolled.
For clinical scores, Chromameter® readings and product comparison, the mean, standard deviation, and 95% confidence interval were calculated at each visit, along with significance for change from previous visit, and from baseline, using Student's paired t-test or Wilcoxon signed rank test. Statistical Package for the Social Sciences version 19 was used.
Product efficacy and safety were assessed using the intent-to-treat population, defined as the set of participants who were enrolled in the study.
Results
Patient disposition and demographics
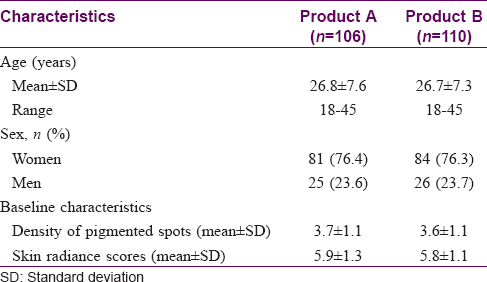
Of the 230 enrolled participants, 115 were randomized to each product; 216 (93.9%) completed the study with loss on follow-up being the major reason for discontinuation [Figure - 2]. Demographic and baseline characteristics were comparable between the groups [Table - 1].
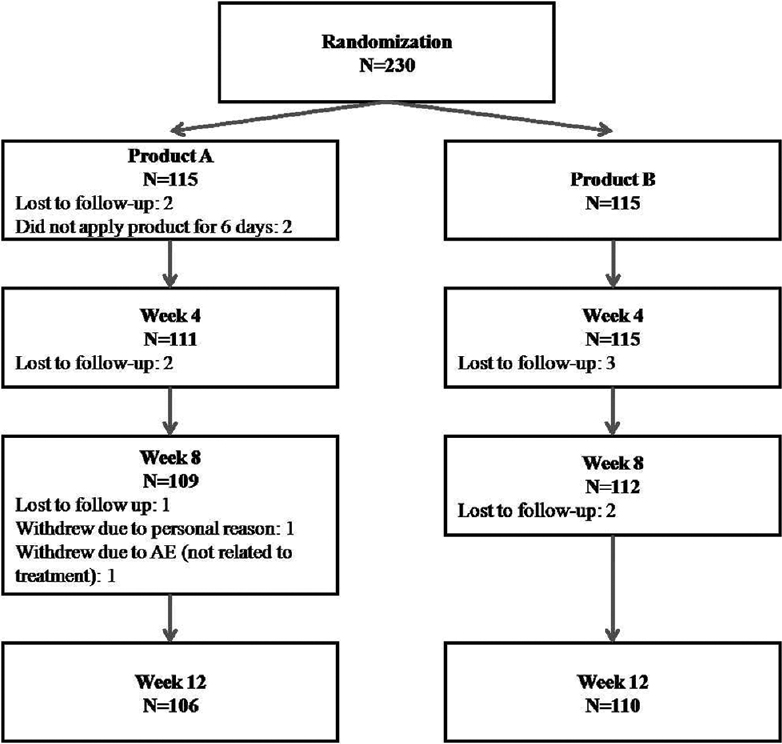 |
| Figure 2: Flow of the number of participants enrolled and completing the study. AE: Adverse event |
Efficacy
Clinical assessment
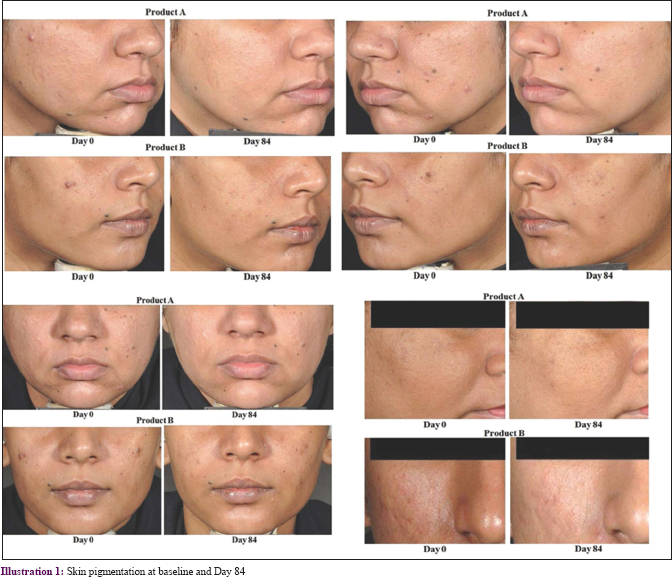
Dermatological assessment of the density of pigmented spots revealed significant reduction in both groups (P < 0.001 at all visits), as compared to baseline [Table - 2] and [Figure - 3] and [Illustration 1]; mean (standard deviation) D84-D0: Product A: −1.6 (0.8), Product B: −1.5 (0.7); % change D84-D0: Product A: 43.1%, Product B: 41.1%] with no between-group significance observed. Further, 94% (100/106) and 93% (100/110) of participants for Products A and B showed improvement, respectively. Similarly, the skin radiance improved significantly in both groups (P < 0.001 at all visits), as compared to baseline [mean (standard deviation) D84-D0: Product A: −2.2 (1.0); Product B: −2.3 (1.0); % change D84-D0: Product A: 37.7%, Product B: 39.9%; [Figure - 3], with 96% (102/106) and 95% (105/110) of participants showing similar improvements (not significantly different) with Products A and B, respectively.

 |
| Figure 3: Density of pigmented spots and skin radiance over time |
Skin color map
The clinician assessment using a skin color map revealed improvements (changes in skin color) as compared to baseline for both groups [Table - 3]; differences between the two groups were not significant.
Assessment of skin color using Chromameter®
L* values remained constant [Figure - 4], as compared to baseline for both groups, except at D28 in the Product B group (P = 0.006), with no significant differences between groups. Also, a significant reduction in a* value indicating reduction in redness (P < 0.001), and a significant increase in b* value indicating a yellower skin or an increased brightness (P < 0.001 at all time points except at D56; P = 0.020 for Product A and P = 0.008 for Product B) were observed. No significant difference between groups was observed for both a* and b* components. Similarly, individual typological angle values were not significant for both products at all the time points, as compared to baseline, except at D84 for Product A (P = 0.04); no significant difference between groups was observed.
 |
| Figure 4: L*, a*, b* and individual typological angle values on cheek at different time points with error bars, 95% confidence interval |
Self-assessments
L'Oréal skin color chart
Participants perceived a shift in skin color toward lightening with Product A at days 28 and 84, while improvements were observed at only D28 with Product B [Table - 4]. Overall, 55% (58/106) and 53% (58/110) of participants showed improvement at D84 with Products A and B, respectively; no significant difference between groups was observed.
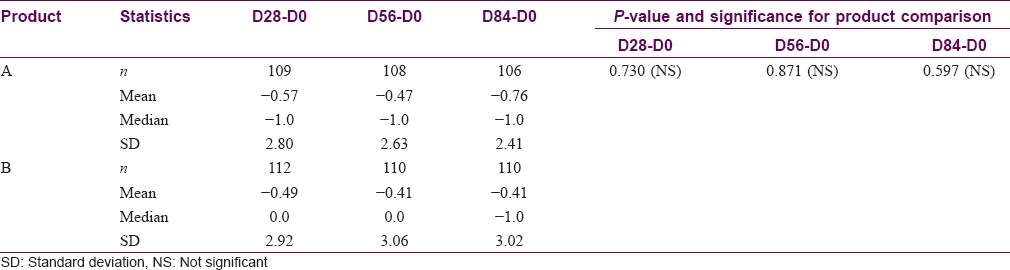
Safety
Overall, 14 adverse events (Product A: 9, Product B: 5) were reported, of mild-to-moderate severity. The most common adverse event was fever (n = 4), unrelated to products, which resolved. No serious adverse events were reported. Local intolerance events were isolated or intermittent, the most common being a burning sensation (Product A: 5; Product B: 4), that resolved spontaneously.
Discussion
Skin changes induced by sun exposures are often mitigated by constitutive pigmentation, although the latter does not provide complete protection against ultraviolet rays. An appropriate protection against solar ultraviolet exposure is required during daily activities to prevent clinical, cellular and molecular changes that may lead to photodamage, including photoaging, sunburn, uneven skin tone, pigmentation and cutaneous malignancy,[12],[20],[21] by a) avoiding sun exposure during the peak hours of ultraviolet radiation (i.e., between 12 and 3 PM); b) utilizing ultraviolet protective clothing, hats, sunglasses and c) frequent applications of appropriate sunscreen depending on the intensity of sun radiance exposure and the level of photoprotection used.[12],[20],[21],[22],[23]
Indian people are susceptible to pigmentation disorders.[10],[24] In India, the high incidence of pigmentary disorders, in high altitude and a sun exposed environment, suggests that pigmentary disorders in darker skins are related to ultraviolet exposure.[6],[25],[26] Dark-skinned people generally do not usually use sunscreens, considering themselves as being at a lower risk of sun-related damage. However, data related to prevention of pigmentation by sunscreens on Indian skin types IV and V is scarce.
This was the first such study to evaluate the effect of sunscreen products with different sun protection factors but comparable PA values (Product A: 50 PA+++ and B: 19 PA+++) on Indian skin types IV and V. The results showed a significant improvement from baseline, with a decreased density of pigmented spots throughout the study for both products. Moreover, both the products were at parity regarding density of pigmentation, in agreement with previous studies that showed that a product with a strong Ultraviolet A protection factor offers a real protection against pigmentation even in darker skin tones such as types IV and V.[9],[27] These results are consistent with findings from other comparable studies, which have shown that applications of sunscreens on darker skins protect against pigmentation.[2],[8],[28],[29]
A significant and similar improvement was observed in both skin pigmentation and skin radiance in both groups, but no differences were observed between the two groups. The Chromameter® L* values showed no significant difference from baseline, inferring efficient protection against darkening. The same trend was observed for both products despite 2 h daily exposure to zenithal sun, indicating that both products provide high protection. Assessment of a* value indicating redness of the skin was significantly reduced in both groups on regular usage of the sunscreens. Further, the b* value (the yellow component) increased as compared to baseline and was similar in both groups. Both products were well tolerated with only minor adverse events that were unrelated to the use of products.
Limitations
The study limitations were a short study period (12 weeks) and it being a single-center study.
Conclusions
Although the Indian population with darker phototypes is daily exposed to high solar ultraviolet radiances, this study shows that the regular usage of sunscreen products with moderate sun protection factor and high PA+++ values may offer an efficient protection against pigmentation irregularities and improve the overall skin radiance in Indians with Fitzpatrick phototypes IV and V.
Acknowledgements
The authors thank the study participants, without whom this study would never have been accomplished. This work would not have been achievable without the great contribution and support of Francois Pradier, Director of Research and Innovation, L'Oréal, India, Sandeep Kriplani, General Manager, Active cosmetics division for providing the products, Dr. Urmi Sarkar for data compilation, Muzzammil Hosenally and Rashi Nangia for data analysis. The authors also wish to thank Dr. Venugopal Madhusudhana (Lambda Therapeutic Research Ltd.) for help in drafting the manuscript and submission.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was supported financially by L'Oréal Research and Innovation, Mumbai, India.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Verma SB. Redefining colour of Indian skin. J Eur Acad Dermatol Venereol 2008;22:1263-4.
[Google Scholar]
|
| 2. |
Hourblin V, Nouveau S, Roy N, de Lacharrière O. Skin complexion and pigmentary disorders in facial skin of 1204 women in 4 Indian cities. Indian J Dermatol Venereol Leprol 2014;80:395-401.
[Google Scholar]
|
| 3. |
Sachdeva S. Fitzpatrick skin typing: Applications in dermatology. Indian J Dermatol Venereol Leprol 2009;75:93-6.
[Google Scholar]
|
| 4. |
Liu J, Zhang W. The influence of the environment and clothing on human exposure to ultraviolet light. PLoS One 2015;10:e0124758.
[Google Scholar]
|
| 5. |
Battie C, Jitsukawa S, Bernerd F, Del Bino S, Marionnet C, Verschoore M, et al. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol 2014;23 Suppl 1:7-12.
[Google Scholar]
|
| 6. |
Singh G, Chatterjee M, Grewal R, Verma R. Incidence and care of environmental dermatoses in the high-altitude region of Ladakh, India. Indian J Dermatol 2013;58:107-12.
[Google Scholar]
|
| 7. |
Bernerd F, Asselineau D. An organotypic model of skin to study photodamage and photoprotection in vitro. J Am Acad Dermatol 2008;58:S155-9.
[Google Scholar]
|
| 8. |
Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol 2010;130:2092-7.
[Google Scholar]
|
| 9. |
Marionnet C, Tricaud C, Bernerd F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int J Mol Sci 2014;16:68-90.
[Google Scholar]
|
| 10. |
Nouveau S, Agrawal D, Kohli M, Bernerd F, Misra N, Nayak CS, et al. Skin hyperpigmentation in Indian population: Insights and best practice. Indian J Dermatol 2016;61:487-95.
[Google Scholar]
|
| 11. |
Del Bino S, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol 2013;169 Suppl 3:33-40.
[Google Scholar]
|
| 12. |
Bernerd F, Moyal D, Pai SB, Srinivas CR. Ultraviolet-induced skin damage and its prevention with sunscreen. In: Basic Science for Modern Cosmetic Dermatology. 1st ed.. New Delhi: Jaypee Brothers Medical Publishers; 2014. p. 91.
[Google Scholar]
|
| 13. |
Sharma VK, Sahni K, Wadhwani AR. Photodermatoses in pigmented skin. Photochem Photobiol Sci 2013;12:65-77.
[Google Scholar]
|
| 14. |
Srinivas CR, Sekar CS, Jayashree R. Photodermatoses in India. Indian J Dermatol Venereol Leprol 2012;78 Suppl 1:S1-8.
[Google Scholar]
|
| 15. |
Francoise B, Dominique M, Pai SB, Chakravarthi S. Ultraviolet induced skin damage and its prevention with sunscreen. In: Basic Science for Modern Cosmetic Dermatology. 1st ed.. New Delhi: Jaypee Brothers Medical Publishers; 2015. p. 91-113.
[Google Scholar]
|
| 16. |
Sharma L, Basnet A. A clinicoepidemiological study of polymorphic light eruption. Indian J Dermatol Venereol Leprol 2008;74:15-7.
[Google Scholar]
|
| 17. |
Bazin R, Flament F. Pigmentary homogeneity scales. In: Skin Aging Atlas Asian Type. 1st ed.., Vol. 2. Paris: Med'Com Editions; 2010.
[Google Scholar]
|
| 18. |
Hourblin V, Cointereau-Chardon S, Misra N, Flament F, Nouveau S, Vedamurthy M, et al. Skin color types and Indian skin characteristics. In: Srinivas C, Verschoore M, editors. Basic Science for Modern Cosmetic Dermatology. 1st ed.. New Delhi: Jaypee Brothers; 2015. p. 47-61.
[Google Scholar]
|
| 19. |
De Rigal J, Abella ML, Giron F, Caisey L, Lefebvre MA. Development and validation of a new skin color chart. Skin Res Technol 2007;13:101-9.
[Google Scholar]
|
| 20. |
Yam JC, Kwok AK. Ultraviolet light and ocular diseases. Int Ophthalmol 2014;34:383-400.
[Google Scholar]
|
| 21. |
Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J 2003;17:1177-9.
[Google Scholar]
|
| 22. |
Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: Differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol 2007;6:10-6.
[Google Scholar]
|
| 23. |
Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol 2008;58:S160-6.
[Google Scholar]
|
| 24. |
Al-Jamal MS, Griffith JL, Lim HL. Photoprotection in ethnic skin. Dermatol Sin 2014;32:217-24.
[Google Scholar]
|
| 25. |
Taylor SC, Cook-Bolden F, Rahman Z, Strachan D. Acne vulgaris in skin of color. J Am Acad Dermatol 2002;46:S98-106.
[Google Scholar]
|
| 26. |
Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol 2000;39:57-106.
[Google Scholar]
|
| 27. |
Fourtanier A, Moyal D, Seité S. Sunscreens containing the broad-spectrum UVA absorber, mexoryl SX, prevent the cutaneous detrimental effects of UV exposure: A review of clinical study results. Photodermatol Photoimmunol Photomed 2008;24:164-74.
[Google Scholar]
|
| 28. |
Flament F, Bazin R, Laquieze S, Rubert V, Simonpietri E, Piot B, et al. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin Cosmet Investig Dermatol 2013;6:221-32.
[Google Scholar]
|
| 29. |
Kollias N, Malallah YH, Al-Ajmi H, Baqer A, Johnson BE, González S, et al. Erythema and melanogenesis action spectra in heavily pigmented individuals as compared to fair-skinned Caucasians. Photodermatol Photoimmunol Photomed 1996;12:183-8.
[Google Scholar]
|
Fulltext Views
10,989
PDF downloads
4,316





