Translate this page into:
A rapidly growing crateriform nodule on the nose
Correspondence Address:
Ossama Abbas
Department of Dermatology, American University of Beirut Medical Center, P.O. Box 11-0236, Riad El Solh/Beirut 1107 2020
Lebanon
| How to cite this article: Khalil J, Rahal JA, Abbas O, Maamari M. A rapidly growing crateriform nodule on the nose. Indian J Dermatol Venereol Leprol 2020;86:752 |
Case
A 34-year-old otherwise healthy woman presented for a rapidly growing skin lesion over the nose of 5 weeks' duration. She attempted treatment with topical antibiotics with no improvement. Physical examination revealed a 1.5 cm crateriform erythematous nodule with a central crusted keratinous core over the left nasal sidewall [Figure - 1]. The clinical differential diagnosis mainly included epithelial neoplastic tumors such as keratoacanthoma or infections such as cutaneous leishmaniasis, atypical mycobacterial infection or deep fungal infection.
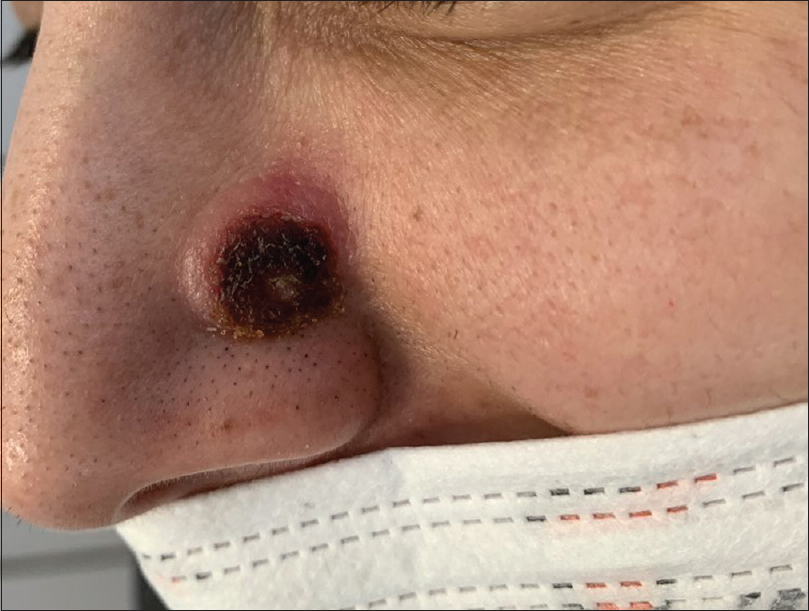 |
| Figure 1: A crateriform erythematous nodule with a central crusted keratinous core over the left nasal groove |
A biopsy specimen [Figure - 2] and [Figure - 3] was taken and revealed irregular epidermal hyperplasia, and a dense dermal mixed cell infiltrate consisting of numerous neutrophils, small lymphocytes, and numerous large, atypical and mitotically active lymphoid cells. These atypical cells exhibited large, pleomorphic, vesicular and hyperchromatic nuclei with prominent nucleoli and abundant cytoplasm [Figure - 2]. Immunohistochemical staining [Figure - 3] of these cells showed positivity for CD3, CD4, lymphocyte-associated antigen and CD30 (more than 75% of tumor cells) with negative staining for CD15, CD20 and anaplastic lymphoma kinase. Ki-67 revealed high proliferative index. Special stains and skin tissue cultures were negative for infectious etiology.
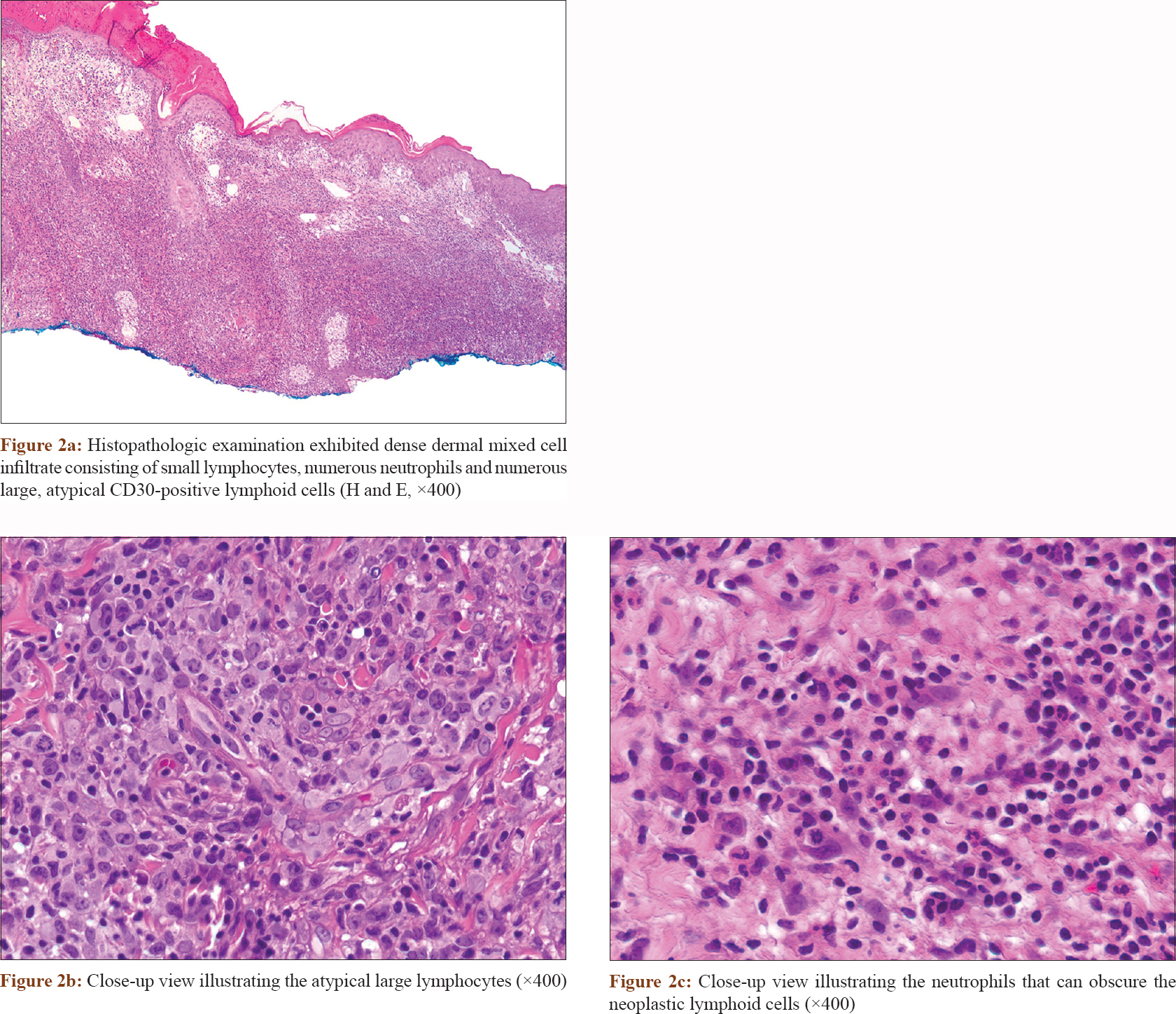 |
 |
What is Your Diagnosis?
Diagnosis
Primary cutaneous anaplastic large cell lymphoma
Discussion
Primary cutaneous anaplastic large cell lymphoma is grouped into the spectrum of the primary cutaneous CD30+ lymphoproliferative disorders, constituting the second most common form of primary cutaneous T-cell lymphoma following mycosis fungoides and accounting for 25% of cases. It is an indolent neoplasm with a 5-year survival of up to 95%. It predominantly affects adults (age range 50-70 years) and has a male predominance (male to female ratio 2:1).[1],[2]
Clinically, it typically presents with a solitary nodule or tumor which often ulcerates. Multifocal involvement has also been described. Lesions may spontaneously regress, indicating a favorable prognosis; however, relapses are common.[2] Histologically, the tumors cells are usually atypical, pleomorphic, irregularly shaped with prominent nucleoli, abundant basophilic cytoplasm and high mitotic index. These cells comprise at least 30% of the inflammatory cell infiltrate. Epidermotropism, adnexal and lymphatic involvement may also be seen. The majority of the neoplastic cells express CD2, CD3 and CD30 (at least 75% of the tumor cells). Positive immunohistochemical staining with anaplastic lymphoma kinase was previously considered in favor of systemic anaplastic large cell lymphoma and against the diagnosis of primary cutaneous anaplastic large cell lymphoma; however, anaplastic lymphoma kinase positivity has recently been well documented in rare cases of primary cutaneous anaplastic large cell lymphoma.[2],[3]
Primary cutaneous anaplastic large cell lymphoma presenting as a clinical mimicker of keratoacanthoma has been reported in the literature. In the cases described, the lesions presented as a rapidly enlarging tumor with a central keratinous plug, similar to our patient's presentation.[4],[5] Furthermore, primary cutaneous anaplastic large cell lymphoma may also mimic squamous cell carcinoma or keratoacanthoma histologically by displaying pseudocarcinomatous hyperplasia. In such cases, the keratinocytes may also exhibit atypia, and the hyperplasia may easily obscure the tumor cells, leading to an erroneous diagnosis.[4],[5] Growth factors secreted by the tumor cells, such as epidermal growth factors and transforming growth factors, are believed to cause this hyperplasia.[5]
Moreover, a neutrophil/eosinophil-rich primary cutaneous anaplastic large cell lymphoma, albeit rare, has also been reported. The largest and most recent case series conducted by Konget al. included a total of 9 patients.[6] They described a neutrophil/eosinophil-rich inflammatory cell infiltrate which was not confined to ulcerated or purulent areas with the absence of infection or necrosis. This constitutes a diagnostic challenge since the dense inflammatory infiltrate may mask the atypical lymphoid cells and lead to an erroneous diagnosis of keratoacanthoma or squamous cell carcinoma.[4] Studies suggest that tumor cells produce interleukins 5 and 8 which lead to the dense neutrophilic and eosinophilic infiltrate.[4]
Though the morphologic and immunophenotypic characteristics of primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis overlap significantly especially lymphomatoid papulosis type C whose microscopic features may be indistinguishable from primary cutaneous anaplastic large cell lymphoma, differentiation can be achieved by correlating the pathologic features with the clinical presentation. Lymphomatoid papulosis is usually characterized by recurrent waxing and waning crops of papules and nodules that tend to be smaller than those of primary cutaneous anaplastic large cell lymphoma. Its other histologic types have histologic features that allow their differentiation from primary cutaneous anaplastic large cell lymphoma. These variants may exhibit wedge-shaped infiltrate of scattered/small clusters of large atypical CD30+ lymphocytes admixed with inflammatory cells in lymphomatoid papulosis type A, epidermotropic infiltrate of small-medium-sized lymphocytes variably expressing CD30 in lymphomatoid papulosis type B, marked epidermotropism of atypical CD8+ and CD30+ lymphocytes in lymphomatoid papulosis type D and angioinvasive atypical CD30+ lymphocytic infiltrates with hemorrhage and necrosis in lymphomatoid papulosis type E.
Treatment options include surgical excision, local radiation and topical bexarotene, mainly for solitary or localized primary cutaneous anaplastic large cell lymphoma. Multifocal or disseminated disease is treated with systemic chemotherapy, photochemotherapy, targeted therapies such as brentuximab (monoclonal antibody to CD30) and even allogeneic and autologous stem cell transplantation with variable outcomes.[2] Systemic work-up in our patient was negative, and the lesion was surgically excised with no recurrence after 3 months of follow-up.
This case illustrates a diagnostic challenge whereby a primary cutaneous anaplastic large cell lymphoma can mimic a keratoacanthoma clinically and display a dense neutrophil-rich inflammatory cell infiltrate histologically. Given the clinical overlap with keratoacanthoma, it is important to consider it in patients with no known risk factors for keratoacanthoma or squamous cell carcinoma presenting with rapidly growing keratoacanthoma-like lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
| 1. |
Kempf W, Kerl K, Mitteldorf C. Cutaneous CD30-positive T-cell lymphoproliferative disorders-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg 2018;37:24-9.
[Google Scholar]
|
| 2. |
Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol 2017;44:570-7.
[Google Scholar]
|
| 3. |
Kumaran MS, Jithendriya M, Nagaraj P, Tirumalae R, Jayaseelan E. Anaplastic lymphoma kinase-positive primary cutaneous anaplastic large cell lymphoma-is it a new variant? Indian J Dermatol Venereol Leprol 2012;78:354-7.
[Google Scholar]
|
| 4. |
Lin JH, Lee JY. Primary cutaneous CD30 anaplastic large cell lymphoma with keratoacanthoma-like pseudocarcinomatous hyperplasia and marked eosinophilia and neutrophilia. J Cutan Pathol 2004;31:458-61.
[Google Scholar]
|
| 5. |
Martín JM, Ricart JM, Monteagudo C, Alcácer J, Pinazo I, Tomás L, et al. Primary cutaneous CD30+anaplastic large-cell lymphomas mimicking keratoacanthomas. Clin Exp Dermatol 2007;32:668-71.
[Google Scholar]
|
| 6. |
Kong YY, Dai B, Kong JC, Lu HF, Shi DR. Neutrophil/eosinophil-rich type of primary cutaneous anaplastic large cell lymphoma: A clinicopathological, immunophenotypic and molecular study of nine cases. Histopathology 2009;55:189-96.
[Google Scholar]
|
Fulltext Views
3,285
PDF downloads
3,091





