Translate this page into:
A study of clinicopathologic profile of 15 cases of hypopigmented mycosis fungoides
Correspondence Address:
Uday Khopkar
Department of Dermatology, Seth GS Medical College and KEM Hospital, Parel, Mumbai-400 012
India
| How to cite this article: Khopkar U, Doshi BR, Dongre AM, Gujral S. A study of clinicopathologic profile of 15 cases of hypopigmented mycosis fungoides. Indian J Dermatol Venereol Leprol 2011;77:167-173 |
Abstract
Background: Mycosis fungoides (MF) is cutaneous lymphoma of the T-cell lineage. Hypopigmented MF is a clinical variant of MF, described mainly in Asians. This is a retrospective clinicopathologic analysis of hypopigmented MF at a tertiary care center. Aims: To describe the clinicopathologic profile of hypopigmented MF. Methods: Records of clinicopathologic notes over a 5-year period ranging from January 2005 up to December 2009 were reviewed over a period of 3 months, of which 15 cases were diagnosed with hypopigmented MF based on clinicopathologic correlation. Results: Hypopigmented MF was found to be more common in males, and between second and fourth decades of life. The latent period between onset and diagnosis was around 3.83 years. Most of the patients were asymptomatic 80% (12/15), with skin changes of subtle atrophy in 46.66% (7/15), scaling in 20% (3/15) and focal changes of poikiloderma in 26.66% (4/15) patients. Most common sites of distribution of the lesions were the trunk and extremities. Many of the cases had been clinically mistaken for Hansen's disease prior to correct diagnosis. Marked epidermotropism and tagging of epidermis by large lymphocytes characterizes the condition histopathologically. Of the 15 cases, immunohistochemistry was possible in 10 cases, of which 8 showed predominant CD8 positive epidermotropic infiltrates and two cases showed absence of CD8 positive and CD4 positive lymphocytic infiltrate in the epidermis. Conclusion: Hypopigmented MF presents as hypopigmented asymptomatic patches without any erythema or infiltration in its early stage and mimics Hansen's disease. Skin biopsy clinches the diagnosis.Introduction
Hypopigmented mycosis fungoides (MF) is an underrecognized variant of MF characterized by hypopigmented patches and has been described mainly in children and dark skinned individuals, especially Asians. [1] Clinically, it may resemble other dermatoses like postinflammatory hypopigmentation, early vitiligo and patches of borderline tuberculoid Hansen′s disease. [1] A high index of suspicion is needed to make a diagnosis of this condition.
Diagnosis of early stage of MF is usually based on clinicopathologic correlation. The lack of resources or unavailability of confirmatory tests and lack of sensitivity of tests like T-cell receptor gene re-arrangement studies on skin and blood in early stages make the diagnosis of patch stage MF a difficult proposition histopathologically and immunologically. [1] Also, the algorithm for diagnosis of classic presentations of early MF is not applicable for atypical clinical and histologic variants including hypopigmented MF, follicular and purpuric or palmoplantar MF. [2] Hence, the diagnosis of MF has to be reached based on clinical and histopathological correlation. Several studies have reported a latent period of 4-10 years from the onset of disease to definitive diagnosis. There have been a few solitary case reports of hypopigmented MF from India. However, we came across 15 such cases in our setup over the last 5 years. We herewith give in detail the clinicopathologic profile of those patients who presented to our department over the last 5 years.
Methods
Clinicopathologic notes of all biopsies done during the last 5 years (8500) at our department were reviewed over a period of 3 months. Of these, 45 cases were diagnosed as classic MF and 15 cases were diagnosed as hypopigmented MF based on clinicopathologic correlation. In all of these cases, at least two biopsies had been performed at the same or different points of time (except for two children in whom a biopsy could not be repeated). In cases suspected of Hansen′s disease clinically, Fite Faraco staining was done in addition to H & E staining. A 4 mm punch biopsy was done in all patients after taking an informed consent for the same. All patients were investigated at the time of diagnosis with complete blood count, peripheral smear, buffy coat smear, ultrasonography of abdomen and pelvis, and X-ray chest. Immunohistochemistry for CD4 and CD8 positivity was done whenever possible. The demographic parameters and histopathology of these 15 cases were analyzed and are summarized in [Table - 1].
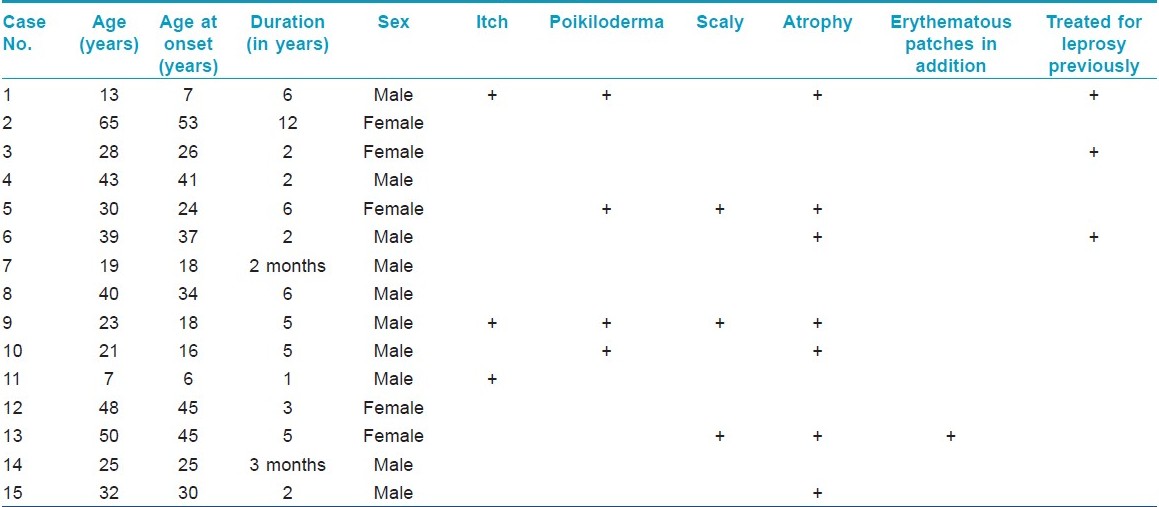
Results
The incidence of hypopigmented MF was found to be 0.17 per 100 biopsies (15/8500). The incidence of hypopigmented MF amongst the total number of patients diagnosed to have MF was 30% (15/45). Hypopigmented MF was found to be more common in males, i.e. 66.66% (10/15). Fifty-three percent (8/15) of the patients diagnosed belonged to the second to fourth decade of life. Average age at the time of diagnosis was 32.2 years with the age range being 7-65 years.
The average latent period between onset and diagnosis was around 3.83 years (range 2 months-12 years). Most common sites of distribution of the lesions were the trunk [Figure - 1] and back (80%) and proximal extremities (arms 80% and thighs 54%) [Figure - 2] and [Figure - 3]. Most of the patients (80%; 12/15) were asymptomatic, with some of the lesions showing skin changes of subtle atrophy in 46.66% (7/15), scaling in 20% (3/15), and changes of hyperpigmentation with hypopigmentation and few telangiectasia were seen in a one or two patches along with classic hypopigmented patches in 26.66% (4/15) patients [Figure - 4]. Ten out of fifteen (66.6%) cases had an initial differential diagnosis of Hansen′s disease while 20% of the cases had taken multidrug therapy for Hansen′s disease previously. In four patients, poikilodermatous changes over patches of hypopigmentation were present, though the hypopigmented patches predominated.
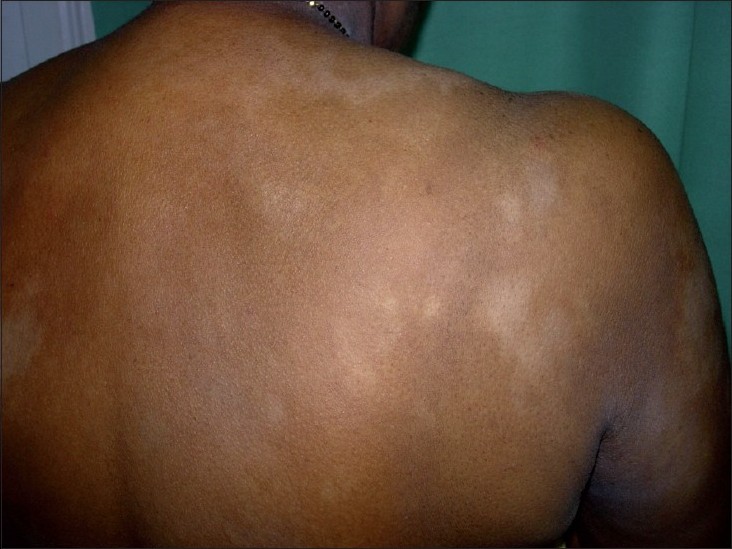 |
| Figure 1: Multiple hypopigmented, ill-defined to well-defined patches on the back |
 |
| Figure 2: Ill-defined to well-defined hypopigmented patches with slight atrophy on the arm |
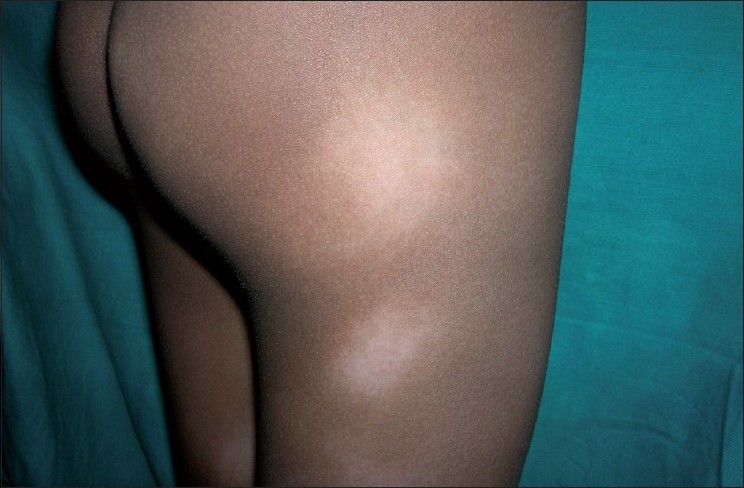 |
| Figure 3: Ill-defined hypopigmented patches over the buttock and thigh in a child |
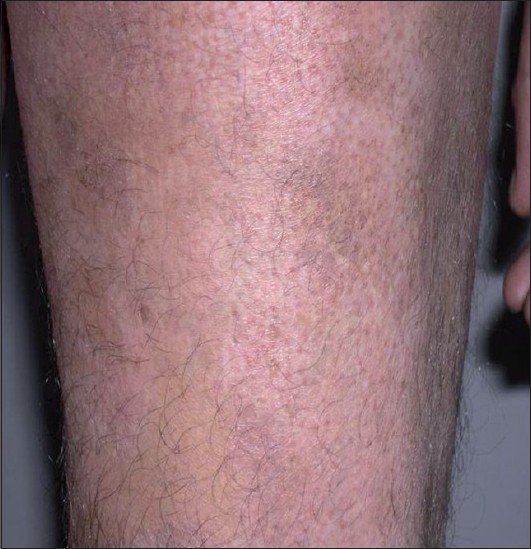 |
| Figure 4: Hypopigmented patches with perifollicular pigmentation and slight atrophy on the thigh |
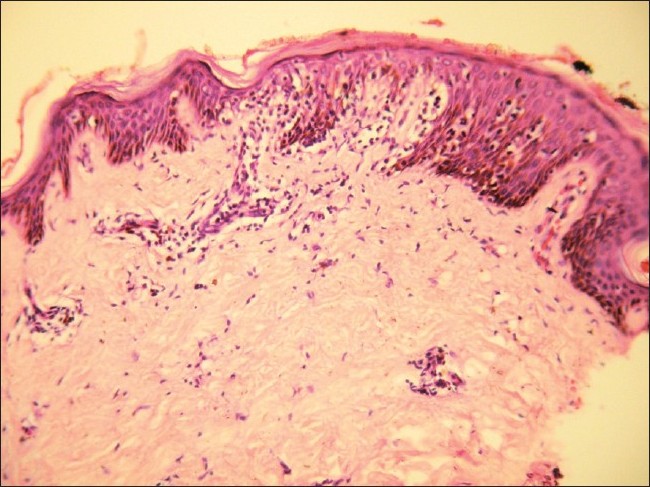
Histopathology showed epidermal atrophy in 26.66% cases (4/15) and epidermal hyperplasia in 40% cases (6/15). Presence of lymphocytes at the dermoepidermal junction [Figure - 5] was the most frequent finding seen in 100% cases (15/15). Large lymphocytes in the epidermis [Figure - 6] were evident in 93.33% cases (14/15). Lymphocytes were found in the epidermis at the upper levels without much spongiosis as compared to the amount of infiltration in 93.33% cases (14/15). Uncommonly, lymphocytes may be arranged in clusters in the epidermis (Pautrier′s microabcesses), which was seen in 40% cases (6/15) [Figure - 7]. Thickened or wiry collagen bundles were noted in 60% cases (9/15). Dermal infiltrate was patchy lichenoid in 13.33% (2/15) cases, superficial perivascular and interstitial in 13.33% (2/15) cases, and sparse superficial perivascular infiltrate in 73.33% (11/15) cases. Telangiectasia or dilated superficial dermal blood vessels were observed in 26.66% (4/15) cases. Frequently, more lymphocytes were present in the epidermis (epidermotropism) than in the dermis (sparse perivascular infiltrate) at times leading to a "normal skin" impression on scanner view. Adnexotropism in the form of follicular involvement was seen in one case. Another feature which we noticed was the presence of melanin incontinence seen in 46.66% of our cases (7/15).
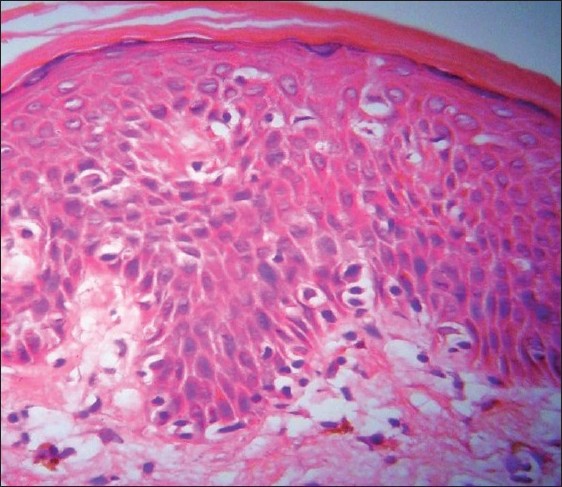 |
| Figure 5: Large atypical lymphocytes with hyperchromatic nuclei and perinuclear halo seen in the epidermis along with tagging of dermoepidermal junction by atypical lymphocytes and melanin incontinence in the upper dermis on (H and E, ×40) |
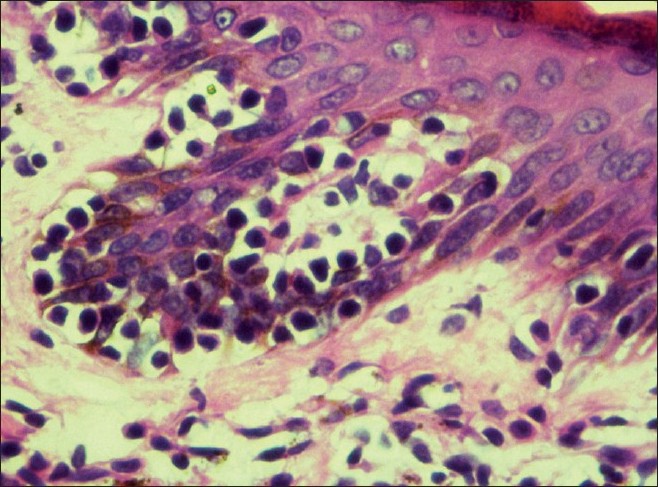 |
| Figure 6: Large lymphocytes in the epidermis and melanin incontinence in the upper dermis on (H and E, ×40) |
 |
| Figure 7: Tagging of lymphocytes along dermoepidermal junction with Pautrier's microabscess on (H and E, ×10) |
Immunohistochemistry for CD4 and CD8 positivity was done in 10 out of 15 cases. Eighty percent (8/10) cases showed predominant epidermotropism by CD8 positive T lymphocytes [Figure - 8], whereas two cases showed no evidence of epidermotropic CD8 positive or CD4 positive lymphocytic infiltrate. Moderate CD4 positive T lymphocyte epidermotropism was observed in five cases and mild CD4 positive epidermotropism was seen in the remaining three cases. In two of our patients who had both hypopigmented patches along with classic patch of MF, immunohistochemistry showed absence of CD8 or CD4 positive epidermotropic cells in hypopigmented patches, while the immunohistochemistry done on the biopsy taken from classic mycosis lesions showed mixed CD4 positive and CD8 positive lymphocytic infiltrate in the epidermis. Hence, on the basis of presence of other clinically characteristic lesions and poikilodermatous changes, these two cases were diagnosed as hypopigmented MF.
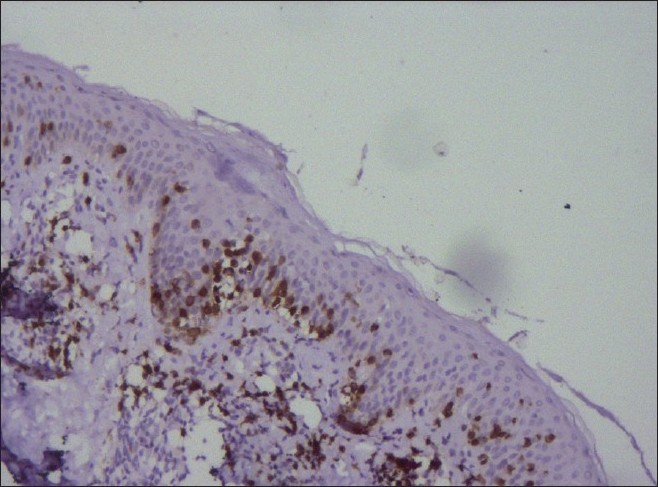 |
| Figure 8: Numerous CD8 positive T lymphocytes in the epidermis on immunohistochemistry study (×10) |
Discussion
In the year 1973, the term "hypopigmented mycosis fungoides" was mentioned for the first time by Ryan et al. [3] He and his co-workers also proposed a hypothesis on the "epidermal origin" of MF, suggesting that foci of abnormal cells might begin to proliferate and spread within the epidermis, first provoking an inflammatory response in the dermis and only later colonizing it. They noted "early involvement of the basal layer of the epidermis" and indicated that it was responsible for "pigmentary changes" that could be seen in the course of the disease, such as hypopigmentation alone and hypopigmentation as a component of poikiloderma. They proposed that it may be due to the destruction of melanocytes in affected skin by neoplastic and non-neoplastic T lymphocytes [3] or the defect in transfer of melanosomes from melanocytes to keratinocytes. Ultrastructural studies have revealed focal invasion of the epidermis by mycosis cells, with degenerative changes in adjacent melanocytes and keratinocytes. [4]
Clinically, the hypopigmented patches are asymptomatic, not sharply circumscribed, and have slightly dry surface. Mild atrophy may be appreciated only on close examination with a lens. Some of these patches may develop focal hyperpigmentation resulting in early poikilodermatous changes in the center as chronicity of infiltration increases.
Monoclonality of the T lymphocytes can be detected with the help of T-cell receptor gene rearrangement studies. However, unlike classical MF which usually shows CD4 positive T cells, many patients with hypopigmented MF show CD8 positive T cells within the epidermis. [5]
A study by Shabrawi-Caelen et al,[6] reported the occurrence of cytotoxic immunophenotype (CD8 positive) in 60% of patients with hypopigmented MF, with a higher percentage (100%) in children as compared to adults and not associated with a worse prognosis. However, in our study, we found 80% of the tested cases to have CD8 positive epidermotropic infiltrate.
Though in some studies CD8 positive MF has been reported to be more aggressive than CD4 positive disease, it may reflect a failure to distinguish CD8 positive MF from other types of primary cutaneous T-cell lymphomas (CTCLs). [7],[8],[9] It has been reported that CD8 positive MF follows a more aggressive course if the neoplastic cells are CD2 negative and CD7 positive. [10] A recent study on prognostic implications of marker studies in MF done by Massone et al,[11] reports lack of significance of marker studies in determining the prognosis of MF.
In our study, all 15 patients were Asians with dark skin type, similar to the findings in the study by Tan et al,[12] that consisted of three Chinese, four Malays, and one Indian, whereas the study by Wain et al, [13] revealed 4 Asian, 3 African Caribbean (dark skinned) and 1 Caucasian (fair skinned) patient. All seven patients in a study by Lambroza et al, [14] too showed predilection for persons with brown or black skin [Table - 2].
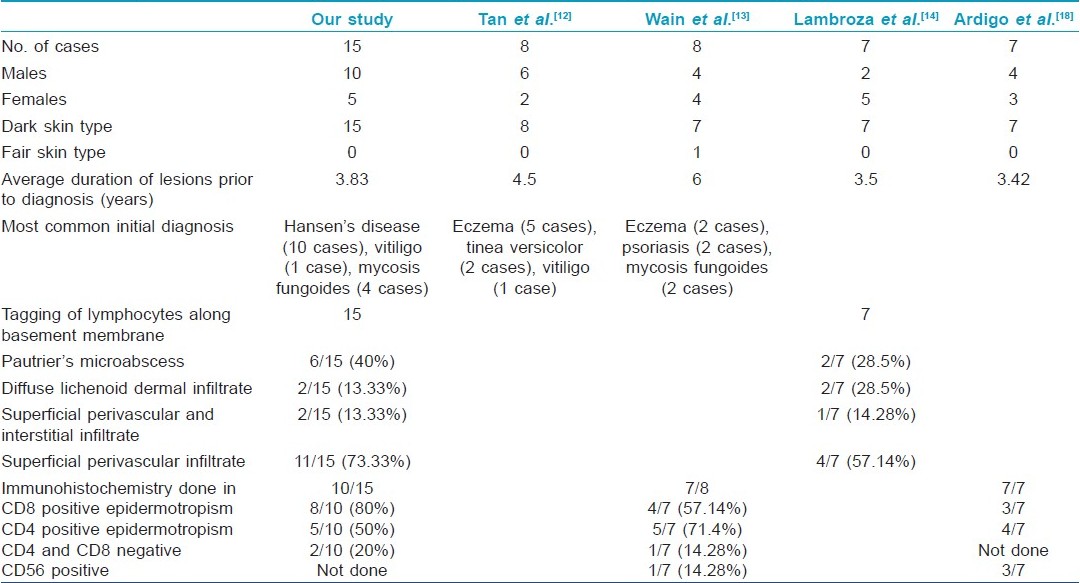
The average age at the time of diagnosis in our study was 32.2 years. The mean age at diagnosis was 35.9 years in the study by Lambroza et al. [14] In a representative study of MF, the age of patients ranged between 17 and 87 years at the time of diagnosis, with average age being 51.8 years. [15] Hence, our observation supports the occurrence of early onset of lesions of hypopigmented MF as compared to typical forms of MF. [15],[16]
On histopathologic examination, the most consistent (100%) finding in our patient was scattered lymphocytes in the basal epidermis similar to the findings seen in all seven cases in the study by Lambroza et al., [14] Forty percent (6/15) of our cases showed evidence of Pautrier′s microabscess, whereas 28.5% (2/7) cases showed presence of Pautrier′s microabscesses in the study by Lambroza et al. [14] The occurrence of melanin incontinence was seen in 46.6% of our cases. Ultrastructural studies have revealed focal invasion of the epidermis by mycosis cells with degenerative changes in adjacent melanocytes and keratinocytes, which explains the occurrence of melanin incontinence and hypopigmentation in hypopigmented MF. [3]
The occurrence of telangiectasia on histopathology was observed in 26.66% (4/15) cases in those patients in whom few of the hypopigmented patches showed early changes of hyperpigmentation and slight atrophy. These changes of early poikiloderma were seen in patients in addition to multiple hypopigmented patches occurring as the predominant lesions in our patients.
The immunohistochemistry finding in our study showed 80% of the patients tested to have cytotoxic CD8 positive lymphocytes in the epidermis, which is far more than that observed by Wain et al.,[13] (57.6%). Two of our patients who showed the presence of both hypopigmented patches as well as classic patches of MF showed no evidence of either CD8 or CD4 positive infiltrate on immunohistochemistry of the hypopigmented lesion. Wain et al, also observed a single case of hypopigmented MF with negative immunohistochemistry finding for CD4 and CD8 lymphocytes in their study.
The most common initial differential diagnosis made in patients in our study was that of Hansen′s disease (10 cases) followed by hypopigmented MF in 4 cases and post-inflammatory hypopigmentation in 2 cases. Clinically, hypopigmented MF can be differentiated from maculo-anesthetic Hansen′s disease by preserved sensations on the patch, no thickening of the peripheral nerves, sparing of the face and distal extremities (sun-exposed areas) and presence of hypopigmentation, atrophy or poikilodermatous changes in some of the lesions. Histologically, it differs from maculo-anesthetic Hansen′s disease by the absence of deep or perineural infiltration and presence of the above described histopathologic picture and infiltrate of CD8 negative T lymphocytes.
Hypopigmented MF can be differentiated from evolving vitiligo by the presence of surface changes like scaling or poikiloderma clinically and by normal number of melanocytes and presence of large lymphocytes in the epidermis histopathologically. Wood′s lamp examination is also helpful.
Hypopigmented MF can mimic progressive macular hypomelanosis (PMH) clinically as it presents as aymptomatic, ill-defined, nummular, non-scaly hypopigmented spots on the trunk, rarely involving proximal extremities and neck/head region with no history of preceding inflammation. [17] It is more commonly noticed in young Black females residing in the tropics. However, it may remit spontaneously after mid-life. It can be differentiated from hypopigmented MF on histology which is characterized by diminished pigment in the epidermis and a normal looking dermis and absence of epidermotropic lymphocytic infiltrates.
The patients in our study showed lack of lymph node enlargement and visceral affection at the time of diagnosis and none of these cases so far have progressed to the plaque or the tumor stage over the past 5 years. Hence, it corroborates the observations of previous studies that hypopigmented variant appears to confer a better prognosis than classical MF.
Thus, it seems to have a favorable, indolent course. However, long-term survival data are not available. Hypopigmented MF in adults and children shows response to treatment with topical corticosteroids, UVB, PUVA, topical nitrogen mustard, or topical carmustine.
Conclusion
In our patients, we found male preponderance in younger age group (mean age 32.2 years). The insidious progress of hypopigmented MF and evolution of it to focal poikilodermatous changes in some of its lesions reflects its good prognosis.
Besides the characteristic histopathologic features of tropism of large lymphocytes for the dermoepidermal junction and unusually sparse dermal lymphocytic infiltrate, features of melanin incontinence were also noted. Though majority of these patches showed epidermotropism by cytotoxic CD8 positive T lymphocytes, the disease remained indolent over a long period of time.
It may present as hypopigmented asymptomatic or atrophic or scaly patches of varying sizes and may mimic a variety of conditions. Persistent hypopigmented lesions should arouse a high index of suspicion for this condition. [18] A lag in its diagnosis may occur due to misdiagnosis or underrecognition of this condition. [12] Most of the cases of hypopigmented MF in our series needed to be differentiated from the hypopigmented patches of borderline tuberculoid leprosy which is endemic and much more prevalent in our region.
Hypopigmented MF probably represents the earliest stage in evolution of MF. [19] Therefore, it may be conjectured that a similar hypopigmented "stage" of MF may be occurring in the white/fair skinned patients but is not being easily noticed as it is asymptomatic and may go undetected. Hence, it may represent an artifact of early detection in persons with darkly pigmented skin.
| 1. |
Inchara YK, Rajalakshmi T. Early mycosis fungoides vs. inflammatory mimics: How reliable is histology? Indian J Dermatol Venereol Leprol 2008;74:462-6.
[Google Scholar]
|
| 2. |
Sarveswari KN, Yesudian P. The conundrum of parapsoriasis versus patch stage of mycosis fungoides. Indian J Dermatol Venereol Leprol 2009;75:229-35.
[Google Scholar]
|
| 3. |
Ryan EA, Sanderson KV, Bartak P, Samman PD. Can mycosis fungoides begin in the epidermis? A hypothesis. Br J Dermatol 1973;88:419-29.
[Google Scholar]
|
| 4. |
Breathnach SM, McKee PH, Smith NP. Hypopigmented mycosis fungoides: Report of five cases with ultrastructural observations Br J Dermatol 2006;106:643-9.
[Google Scholar]
|
| 5. |
Werner B, Brown S, Ackerman AB. "Hypopigmented mycosis fungoides" is not always mycosis fungoides! Am J Dermatopathol 2005;27:56-67.
[Google Scholar]
|
| 6. |
Shabrawi-Caelen L, Cerroni L, Medeiros LJ, McCalmont TH. Hypopigmented mycosis fungoides: Frequent expression of a CD8_ T-cell phenotype. Am J Surg Pathol 2002;26:450-7.
[Google Scholar]
|
| 7. |
Peters MS, Thibodeau SN, White JW Jr, Winkelmann RK. Mycosis fungoides in children and adolescents. J Am Acad Dermatol. 1990;22:1011-8.
[Google Scholar]
|
| 8. |
Lu D, Patel KA, Duvic M, Jones D. Clinical and pathological spectrum of CD8-positive cutaneous T-cell lymphomas. J Cutan Pathol 2002;29:465-72.
[Google Scholar]
|
| 9. |
Agnarsson BA, Vonderheid EC, Kadin ME. Cutaneous T cell lymphoma with suppressor/cytotoxic (CD8) phenotype: Identification of rapidly progressive and chronic subtypes. J Am Acad Dermatol 1990;22:569-77.
[Google Scholar]
|
| 10. |
Lindae ML, Abel EA, Hoppe RT, Wood GS. Poikilodermatous mycosis fungoides and atrophic large-plaque parapsoriasis exhibit similar abnormalities of T-cell antigen expression. Arch Dermatol 1988;124:366-72.
[Google Scholar]
|
| 11. |
Massone C, Crisman G, Kerl H, Cerroni L. The prognosis of early mycosis fungoides is not influenced by phenotype and T-cell clonality. Br J Dermatol 2008;159:881-6.
[Google Scholar]
|
| 12. |
Tan E, Tay YK, Giam YC. Profile and outcome of childhood mycosis fungoides in Singapore. Pediatr Dermatol 2000;17:352-6.
[Google Scholar]
|
| 13. |
Wain EM, Orchard GE, Sean JW, Margaret FS. Outcome in 34 patients with juvenile-onset mycosis fungoides. A clinical, immunophenotypic, and molecular study. Cancer 2003;98:2282-90.
[Google Scholar]
|
| 14. |
Lambroza E, Cohen SR, Phelps R, Lebwohl M, Braverman IM, DiCostanzo D. Hypopigmented variant of mycosis fungoides: Demography, histopathology, and treatment of seven cases. J Am Acad Dermatol 1995;32:987-93.
[Google Scholar]
|
| 15. |
Cohen SR, Stenn KS, Braverman IM. Clinicopathologic relationships, survival and therapy in 59 patients with observations on occupation as new prognostic factor. Cancer 1980;46:2654-66.
[Google Scholar]
|
| 16. |
Epstein EH, Levin DL. Croft JD. Mycosis fungoides: Survival, prognostic features, response to therapy and autopsy findings. Medicine 1972;15:61-72.
[Google Scholar]
|
| 17. |
Relyveld GN, Menke HE, Westerhof W. Progressive Macular Hypomelanosis: An Overview. Am J Clin Dermatol 2007;8:13-9.
[Google Scholar]
|
| 18. |
Ardigó M, Borroni G, Muscardin L, Kerl H, Cerroni L. Hypopigmented mycosis fungoides in Caucasian patients: A clinicopathologic study of 7 cases. J Am Acad Dermatol 2003;49:264-70.
[Google Scholar]
|
| 19. |
Stone ML, Styles AR, Cockerell CJ, Pandya AG. Hypopigmented mycosis fungoides: A report of 7 cases and review of the literature. Cutis. 2001;67:133-8.
[Google Scholar]
|
Fulltext Views
13,172
PDF downloads
4,113





