Translate this page into:
A study to evaluate the efficacy and safety of hydrocortisone aceponate 0.127% lipophilic cream in steroid responsive dermatoses in Indian patients
2 Galderma India Pvt Ltd, Andheri, Mumbai, India
Correspondence Address:
Vijay Baghel
Galderma, India Pvt. Ltd., 23, Steelmade Indl. Estate, Marol, Andheri (E), Mumbai - 400 059
India
| How to cite this article: Mukhopadhyay AK, Baghel V. A study to evaluate the efficacy and safety of hydrocortisone aceponate 0.127% lipophilic cream in steroid responsive dermatoses in Indian patients. Indian J Dermatol Venereol Leprol 2010;76:591 |
Abstract
Background: Topical corticosteroids (CSs) are the mainstay of therapy in various steroid responsive dermatoses. Newer CSs are more efficacious and safer than the older ones. There is no published data on the efficacy and safety of a new steroid hydrocortisone aceponate in the Indian population. Aim: To evaluate the efficacy and safety of hydrocortisone aceponate (0.127%) lipophilic cream in the treatment of steroid responsive dermatoses in Indian patients. Methods: Four hundred and fifteen patients with clinically diagnosed steroid responsive dermatoses enrolled in this study. They were advised to apply hydrocortisone aceponate (0.127%) lipophilic cream as a thin film to all the affected areas twice daily. Cleansing was done prior to the application with either soap-free cleanser or soap (that would not affect the study result). Use of oral antihistamines and/or antibiotics was permissible. However, other oral/topical steroid use was not permitted during the study. Patients were evaluated at day 0 and at day 21. Data were recorded regarding clinical improvement and side-effects, if any. They were then analyzed to determine the efficacy and safety of the cream. Results: Physician's global evaluation of therapy showed that lesions were cleared in 82 (22.10%), excellent result in 200 (53.91%), good result in 72 (19.41%), fair response in 15 (4.04%) and no change in 2 (0.54%) patients. There was no history of exacerbation in any patient. Conclusion: The study showed that hydrocortisone aceponate (0.127%) lipophilic cream is an effective therapeutic agent with a very good safety profile in various steroid responsive dermatoses in the Indian patient population.Introduction
The discovery of corticosteroid (CS) and its role in disease management is one of the most important events in the history of medicine. In the year 1935, Kendell described "compound E" (cortisone). In 1948, CS was first used in the management of rheumatoid arthritis at Mayo Clinic. [1] In 1951, Sulzberger and his colleagues first described the use of CS in inflammatory dermatoses. [2] Again, in 1952, Sulzberger described the use of "compound F" or hydrocortisone as the topically effective CS in the management of inflammatory dermatoses. [3]
CS exerts its effect by entering into the cytoplasm of the keratinocytes and other cells present in the epidermis and dermis through stratum corneum by diffusion. There, it binds to a specific receptor, glucocorticoid receptor, and, subsequently, through a complicated pathway, it influences the activity of the cell and its functions. The therapeutic activity of any CS depends primarily on its antiinflammatory activity and antimitotic activity. [4],[5] But, experience with the commonly used fluorinated CS over the years has shown that the high antiinflammatory capacity is equally associated with the enhanced side-effects on the skin and its structure, e.g. skin atrophy. [6] These events lead to the search of a suitable form of CS that would have a high efficacy with a good safety profile. Esterification of the molecule has been shown to be effective in this regard. [7] Hydrocortisone-21-acetate-17-proprionate (hydrocortisone aceponate), which is a mid-potent steroid, is one of the CS molecules developed in this way by double esterification. However, there is no published data on the efficacy and safety of hydrocortisone aceponate in the Indian population. The present study had been undertaken to study the efficacy and safety of hydrocortisone aceponate lipophilic cream in patients with steroid responsive dermatoses.
Methods
This was an open-label, non-comparative study to evaluate the efficacy and safety of hydrocortisone aceponate 0.127% lipophilic cream in steroid responsive dermatoses. Patients were randomly selected following the inclusion and exclusion criteria as mentioned below:
Inclusion criteria
- Male or female subjects aged from 1 to 65 years with a confirmed clinical diagnosis of steroid responsive dermatoses.
- Clinical severity score of at least 6.
- Patients of steroid responsive dermatoses eligible for treatment with mid-potent topical steroid.
- Written informed consent from patients or their guardians if subjects are under 18 years of age.
- Severe steroid responsive dermatoses requiring treatment with a potent or superpotent glucocorticoid.
- Clinical severity score <6.
- Patient requiring treatment with a medication other than the study drug, which, in the opinion of the investigator, could affect the clinical response in a patient.
- Subjects and/or guardian who are unable to communicate or cooperate with the investigator.
- Patients with a history of hypersensitivity to hydrocortisone aceponate or any other ingredients of the formulation.
- Patients who have received glucocorticoids (oral and/or topical) 1 week prior to the study inclusion.
- Females who are pregnant, nursing or planning a pregnancy.
In this study, 415 patients were included after screening as per the inclusion/exclusion criteria. Written informed consent was obtained from them. A detailed history and clinical examination was performed and the clinical severity score was calculated. To calculate the clinical severity score, the involved area were evaluated for erythema, dryness or scaling, pruritus, excoriations, oozing or crusting, etc. These factors were evaluated using a four-point scale, where 0 = none, 1 = mild, 2 = moderate and 3 = severe. The sum total of all evaluable parameters in a particular patient would give the total clinical severity score for that individual. On the very first day of the study (day 0), patients were explained about the method of appropriate application of the prescribed medication. They were asked to come for a follow-up examination at the end of 3 weeks or as and when necessary. They were also asked to contact and/or inform about any undesired action and/or side-effects as soon as noticed or felt.
Patients were asked to apply hydrocortisone aceponate (0.127%) lipophilic cream as a thin film on the affected area twice daily. Use of oral antibiotics and/or oral antihistamines was permissible during the study.
Evaluation was performed at the end of the therapy. The investigator would record the overall response rate of therapy as follows:
- Clear: no signs or symptoms.
- Marked improvement (excellent): 75% or more improvement.
- Definite improvement (good): 50-74% improvement.
- Minimal improvement (fair): 25-49% improvement.
- No change.
- Exacerbation: worsening of signs and/or symptoms.
Finally, all data thus accumulated were subjected to statistical analysis and a final report was prepared on the findings.
Results
Four hundred and fifteen patients (195 males and 220 females) aged between 1 and 65 years (mean age of 24.50 years) suffering from steroid responsive dermatoses were enrolled in this study. Of the 415 patients, 39 (9.39%) were disqualified for protocol violation and five (1.20%) were dropouts. Thus, the final number of patients completing the study was 371 (89.39%). History of concomitant drug therapy on day 0 was found to be present in 376 (90.60%) patients. Most of them were taking either antihistaminics and/or antibiotics. As about the type of diseases, atopic dermatitis comprised the largest group, affecting 144 (38.92%) patients, followed by contact dermatitis (n = 118, 31.89%), seborrhoeic dermatitis (n = 81, 21.89%) and others (n = 27, 7.3%). When clinical examination was carried out, the percentage of body surface area involved was found to be as follows: >75% in six (1.45%), 75-50% in 42 (10.12%), 49-25% in 134 (32.29%) and below 25% in 233 (56.14%) patients. As about the severity of the disease at study entry, disease of moderate severity was found in 320 (86.25%) patients and severe disease was found in 51 (13.75%) patients.
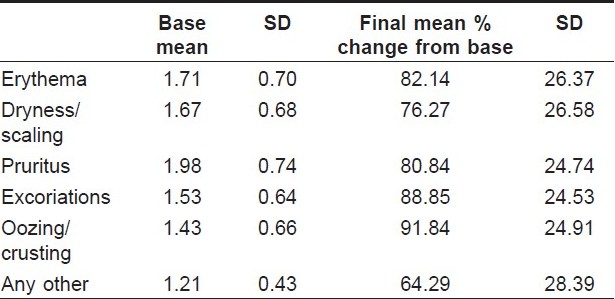
After completion of the study, a comparison between evaluation at day 0 and day 21 was performed. The efficacy of the cream was noted for different parameters. Erythema, dryness or scaling, pruritus, excoriation, oozing and crusting, etc. were improved in a good proportion of the cases [Table - 1]. Physician′s global evaluation of therapy showed that the lesion cleared in 82 (22.10%), had an excellent result in 200 (53.91%), good result in 72 (19.41%), fair response in 15 (4.04%) and no change in two (0.54%) patients. There was no history of exacerbation in any patient. The mean clinical severity score was decreased from 8.94 (2.11 SD) on day 0 to 1.69 (1.73 SD) on day 21. Thus, the percentage change for improvement in signs and symptoms was 81.72% (18.01 SD) from the baseline.
During evaluation of the results, hydrocortisone aceponate lipophilic cream was found to be well tolerated, and most of the adverse events noted were suspected to be due to the concomitant systemic therapy. Of the different adverse events noted, sedation was found in three (0.81%), dry mouth in one (0.27%), abdominal pain in two (0.54%) and local discomfort in the form of mild irritation in two (0.54%) patients, which resolved on its own and did not require withdrawal of the topical therapy.
Discussion
In the present day dermatology, topical glucocorticoids are one of the most frequently prescribed drugs. From the beginning of its usage, it has been noticed that it is a two-edged weapon: increased efficacy is associated with increased side-effects. Therefore, after its initial discovery, many researches were performed to develop molecules with advanced therapeutic benefits with minimal side-effects. Although any topical steroid that fulfills all the criteria of efficacy with safety is yet to be developed, some newly developed CSs like prednicarbate, hydrocortisone aceponate, mometasone furoate, methylprednisolone aceponate, aclometasone dipropionate, etc. met many of the criteria. [8],[9]
A good topical steroid should be able to penetrate the stratum corneum adequately to establish a high concentration locally without raising the serum concentration of the drug. This can be achieved to a great extent by the esterification of the CS and, thereby, increasing its lipophilicity. Hydrocortisone aceponate is one such mid-potent topical steroid with good efficacy and a better safety profile. [8] It is an excellent double-esterified topical CS that can be used to treat various steroid responsive dermatoses. Hydrocortisone aceponate, due to its significant antiinflammatory property and least capacity to induce skin atrophy, can be used to treat some difficult areas like the face, the scrotum and large body areas in children. [10]
The present study showed that hydrocortisone aceponate lipophilic cream is a very effective topical CS cream influencing the erythema, pruritus, scaling, etc., causing minimal adverse reaction in Indian patients. This study has also shown that this preparation may be used in a wide range of patients from the pediatric to the elderly age group with steroid responsive dermatoses [Figure - 1] and [Figure - 2].
 |
| Figure 1 : Efficiency evaluation at day 0 and day 21 |
 |
| Figure 2 : Physician's global evaluation (%) |
Acknowledgment
We wish to acknowledge the following dermatologists for participation in this study:
Dr. Ajita B. Kakkar, Delhi; Dr. Alka Gupta, Delhi; Dr. Ashok P. Wagh, Buldhana; Dr. Bharat M. Shah, Ahmedabad; Dr. Chittambalam P. C., Chennai; Dr. D. K. Sharma, Delhi; Dr. Gaurav Mukhija, Gorakhpur; Dr. Indranil Biswas, Kolkata; Dr. Jayesh Mukhi, Nagpur; Dr. Kiran Godse, Mumbai; Dr. M. Manimegalai, Chennai; Dr. Mailer Ravendran, Erode; Dr. Neeraj Singhal, Meerut; Dr. P. K. Saraswat, Gwalior; Dr. Paramjit Singh Walia, Chandigarh; Dr. Penchala Reddy, Nellore; Dr. R. C. Shah, Mumbai; Dr. Radhakrishnan Nair S., Trivandrum; Dr. Ravindra Wadone, Gadag; Dr. Sharath Kumar, Bangalore; Dr. Shehnaz Arsiwala, Mumbai; Dr. Smita Damle, Pune; Dr. Srabani Ghosh Zoha, Kolkata; Dr. Sunil Sehgal, Delhi; Dr. Sunil Tolat, Pune; Dr. Vani Patalay, Hyderabad; Dr. Vinay Saraf, Mumbai; Dr. Vinay Singh, Delhi; Dr. Vinod P. K., Ernakulam.
| 1. |
Lester RS. Corticosteroids. Clin Dermatol 1989;7:80-97.
[Google Scholar]
|
| 2. |
Sulzberger MB, Witten VH, Yaffe SN. Cortisone acetate administered orally in dermatologic therapy. Arch Dermatol Syphilol 1951;64:573-9.
[Google Scholar]
|
| 3. |
Sulzberger MB, Witten VH. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol 1952;19:101-2.
[Google Scholar]
|
| 4. |
Berth-Jones J. Topical therapy. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 7 th ed. Oxford: Blackwell Science; 2004. p. 5.16-75.21.
th ed. Oxford: Blackwell Science; 2004. p. 5.16-75.21.'>[Google Scholar]
|
| 5. |
Cornell RC, Stoughton RB. The use of topical steroids in psoriasis. Dermatol Clin 1984;2:397-409.
[Google Scholar]
|
| 6. |
Epstein NN, Epstein WI, Epstein JH. Atrophic striae in patients with inguinal intertrigo. Arch Dermatol 1963;87:450-5.
[Google Scholar]
|
| 7. |
Elias PM, Cooper ER, Korc A, Brown BE. Percutaneus transport in relation to stratum corneum structure and lipid composition. J Invest Dermatol 1981;76:297-301.
[Google Scholar]
|
| 8. |
Brazzini B, Pimpinelli N. New and established topical corticosteroids in the dermatology. Am J Clin Dematol 2002;3:47-58.
[Google Scholar]
|
| 9. |
Korting HC, Kerscher MJ, Schδfer-Korting M. Topical glucocorticoids with improved benefit/risk ratio: Do they exist? J Am Acad Dermatol 1992;27:87-92.
[Google Scholar]
|
| 10. |
Mori M, Pimpinelli N, Gianotti B. Topical corticosteroids and unwanted local effects improving the benefit/risk ratio. Drug Saf 1994;10:406-12.
[Google Scholar]
|
Fulltext Views
1,882
PDF downloads
1,514





