Translate this page into:
Adult T cell leukemia-lymphoma
Correspondence Address:
Najeeba Riyaz
Departments of Dermatology and Venereology, Government Medical College, Kozhikode - 673 008, Kerala
India
| How to cite this article: Riyaz N, Abdul Latheef EN, Sasidharanpillai S, Rahima S, Bindu V, Nermin A. Adult T cell leukemia-lymphoma. Indian J Dermatol Venereol Leprol 2014;80:358-361 |
Sir,
Approximately 20 to 30 million people worldwide are infected with human T-lymphotropic virus type I (HTLV 1). [1] Previous reports of adult T-cell leukemia from India lacked confirmation by Western blot test for the presence of HTLV-1 antibodies which is mandatory for the diagnosis. [2] We report a patient with adult T-cell leukemia who presented with pruritic papules and plaques affecting the entire body.
A 70-year-old male presented to the dermatology out-patient department of our hospital with red, raised skin lesions all over the body and scalp with intractable itching. He had these lesions for the past six years, with exacerbations and remissions. His medical history was otherwise non- contributory.
General examination of the patient revealed firm, mobile, non-tender lymphadenopathy involving cervical, axillary and inguinal nodes. Dermatological examination showed erythematous, infiltrated papules and plaques affecting face, trunk [Figure - 1] and limbs with relative sparing of distal extremities. There was no organomegaly.
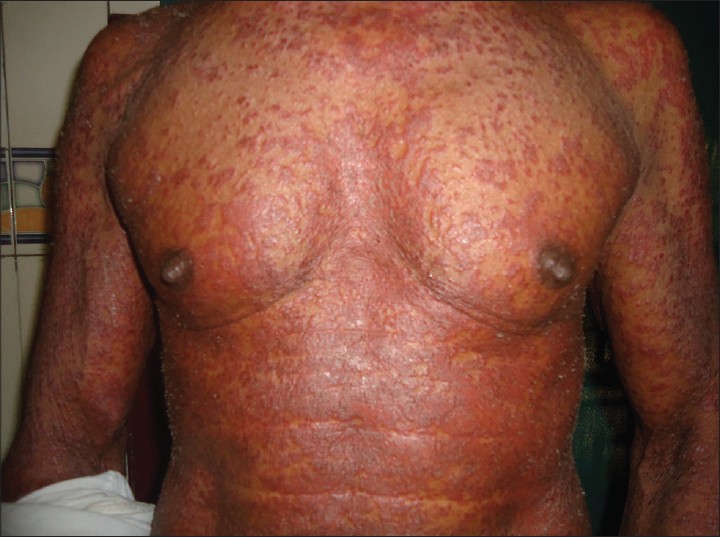 |
| Figure 1: Multiple erythematous papules and plaques on the trunk |
Complete hemogram showed an elevated total count of 18,600/mm 3 and a high erythrocyte sedimentation rate (ESR) of 70 mm/hour. Liver and renal function tests, serum electrolytes, serum calcium, radiography of chest, skull and long bones and ultrasound of abdomen and pelvis were within normal limits. Serology for syphilis, human immunodeficiency virus (HIV) I and II antibodies and antinuclear antibodies were negative. His serum lactate dehydrogenase level was elevated at 560 IU/L. Peripheral smear analysis revealed 42% atypical lymphocytes with large, convoluted nuclei and scanty basophilic cytoplasm [Figure - 2]a. Bone marrow aspirate confirmed these findings [Figure - 2]b. Skin biopsy from an infiltrated papule revealed a dermal infiltrate composed of atypical lymphocytes showing large convoluted hyperchromatic nuclei, scanty cytoplasm and increased mitotic figures and Pautrier′s microabscesses in the epidermis [Figure - 3]. Lymph node biopsy showed atypical lymphocytes with effacement of nodal architecture. Immunohistochemistry of the skin biopsy specimen demonstrated the inflammatory cells to be CD8 and CD20 negative and CD4 [Figure - 4]a and CD25 positive [Figure - 4]b. Flow cytometry analysis of blood confirmed the atypical cells to be CD5, CD3 and CD25 positive and negative for CD10, CD17, CD20 and CD22.
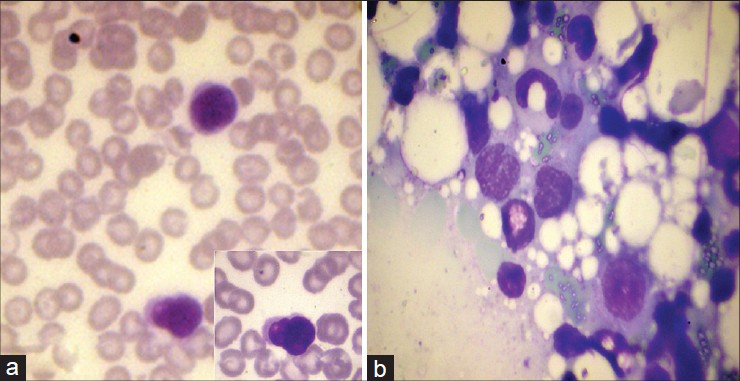 |
| Figure 2: (a) Peripheral smear showing atypical lymphocytes with large, convoluted nuclei and scanty basophilic cytoplasm (Leishman, ×1000). Inset: High power view of the same (Leishman, ×1000) (b) Bone marrow aspirate showing atypical lymphocytes with large, convoluted nuclei and scanty basophilic cytoplasm (Leishman, ×1000) |
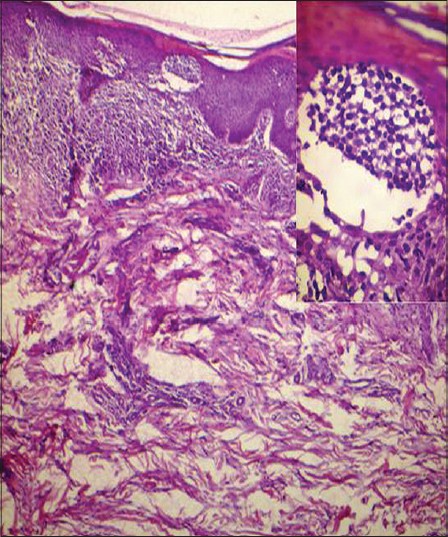 |
| Figure 3: There is a dermal infiltrate of atypical lymphocytes along with a Pautrier's microabscess in the stratum corneum. (H and E, ×40). Inset: High power view of the Pautrier's microabscess. (H and E, ×400) |
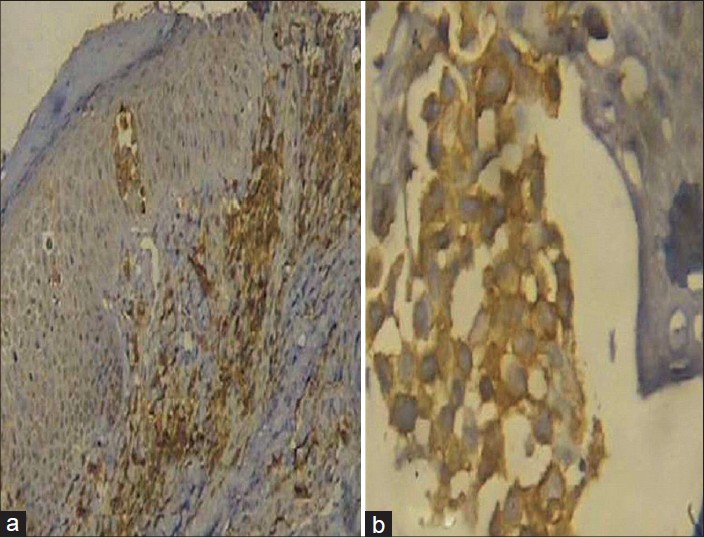 |
| Figure 4: (a) CD4 positive cells in the dermal infiltrate (Immunohistochemistry, DAB Chromogen, ×100) (b) CD25 positive cells in the dermal infiltrate (Immunohistochemistry, DAB Chromogen, ×400) |
With these immunohistochemistry and flow cytometry results, we considered the possibility of adult T cell leukemia/lymphoma (ATLL). The enzyme-linked immunosorbent assay for anti-HTLV-1 antibodies was found to be positive which was subsequently confirmed by Western blot testing. Thus, a final diagnosis of ATLL was made and the patient was referred to the hemato-oncology department where he is now undergoing chemotherapy with a regimen comprising cyclophosphamide, vincristine and prednisolone.
Adult T cell leukemia/lymphoma is a rare and aggressive form of leukemia caused by a retrovirus known as HTLV-1. [3] A higher incidence of the malignancy is documented in areas endemic for HTLV-1 viz. Japan, Africa, South America, Caribbean basin, southern parts of North America and Eastern Europe. [1]
The virus gains entry (a) from mother to child through prolonged breast feeding and less commonly through transplacental transmission; (b) through sexual contact; and (c) through the intravenous route mainly via blood transfusion. [1]
More than 95% of infected individuals remain asymptomatic carriers whereas 1-5% undergo malignant lymphoid proliferation. Environmental factors including other viral infections prevalent in the area play a role in precipitating malignant transformation. [1]
Adult T cell leukemia/lymphoma is diagnosed when a patient with seropositivity for HTLV-1 antibodies develops histologically and/or cytologically proven peripheral T cell malignancy. [3] The characteristic neoplastic cells on cytology are cerebriform or flower cells (activated lymphocytes with convoluted nuclei and basophilic cytoplasm). The most common immunophenotypic profile observed is CD4+/CD8 - lymphocytes as observed in our patient. [4]
The disease can be of four types: acute, lymphoma, chronic and smoldering variants [Table - 1]. [2] Our patient belonged to the category of chronic adult T cell leukemia/lymphoma.
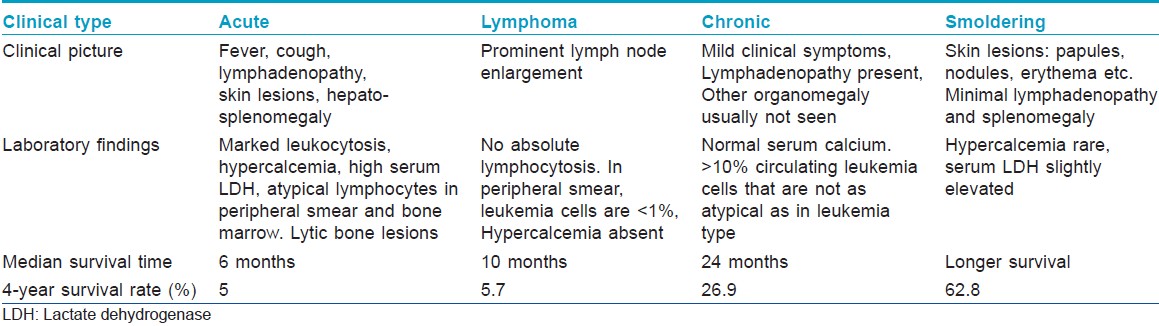
The treatment options range from watchful waiting (in chronic and smoldering types) to chemotherapy, antiviral therapy including zidovudine, allogenic hematopoetic stem cell transplantation and interferon α2 . [3],[5],[6]
Chronic adult T cell leukemia/lymphoma with low serum albumin, or high lactate dehydrogenase (as in our patient) or high blood urea nitrogen concentration is suggested to have an unfavorable prognosis similar to the acute and lymphoma types. [3],[5]
The positive Western blot report in our patient suggests that HTLV-1 is present in our population as well. Screening for the virus in blood donors may help us to assess the prevalence of infection in our area.
We report this case to highlight the possibility of HTLV-1 infection in this region and to stress the importance of surveillance measures. We need to raise awareness regarding this infection which has a potential for malignant transformation.
ACKNOWLEDGMENT
We express our sincere gratitude to Dr Sathi P, Professor and Head, Dept of Pathology, Govt Medical College, Kozhikode and Dr Neena Mampilli, Consultant Pathologist, Baby Memorial Hospital, Kozhikode for their invaluable help in analyzing the histopathology and immunohistochemistry specimens.
| 1. |
Nicot C. Current views in HTLV-1 associated adult T-cell leukemia/lymphoma. Am J Hematol 2005;78:232-9.
[Google Scholar]
|
| 2. |
Khader A, Shaan M, Sasidharanpillai S, Pakran J, Rajan U. Adult T-cell leukemia/lymphoma: A retroviral malady. Indian J Dermatol 2012;57:219-21.
[Google Scholar]
|
| 3. |
Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington Jr W, et al. Definition, prognostic factors, treatment and response criteria of adult T-Cell leukemia-lymphoma: A proposal from an international consensus meeting. J Clin Oncol 2009;27:453-9.
[Google Scholar]
|
| 4. |
Matutes E. Adult T-cell leukaemia/lymphoma. J Clin Pathol 2007;60:1373-7.
[Google Scholar]
|
| 5. |
Gill PS, Harrington W Jr, Kaplan MH, Ribeiro RC, Bennett JM, Liebman HA, et al. Treatment of Adult T-cell leukemia - lymphoma with a combination of interferon alpha and zidovudine. N Eng J Med 1995;332:1744-8.
[Google Scholar]
|
| 6. |
Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, et al. Japan Clinical Oncology Group Study JCOG9801. VCAP-AMP-VECP Compared With Biweekly CHOP for Adult T-Cell Leukemia-Lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 2007;25:5458-64.
[Google Scholar]
|
Fulltext Views
3,571
PDF downloads
2,788





