Translate this page into:
Aggressive angiomyxoma of the vulva in a patient with systemic lupus erythematosus
2 Department of Obstetrics and Gyneacology, All India Institute of Medical Sciences, New Delhi, India
3 Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
B K Khaitan
All India Institute of Medical Sciences, New Delhi 110 029
India
| How to cite this article: Pahwa P, Khaitan B K, Rao A, Kriplani A, Mahey R, Subbarao K C. Aggressive angiomyxoma of the vulva in a patient with systemic lupus erythematosus. Indian J Dermatol Venereol Leprol 2012;78:361-364 |
Abstract
Aggressive angiomyxoma is a rare, slow-growing mesenchymal neoplasm with a tendency to recur. It mainly involves the pelvis, vulva, perineum, vagina, and urinary bladder in adult women of reproductive age group. We describe a 26-year-old female with large swellings of both labia majora which was histologically diagnosed as aggressive angiomyxoma. She also had systemic lupus erythematosus. The swelling was surgically removed and she had no recurrence at 1-year follow-up. Although it is a rare tumor, it must be considered as a differential diagnosis for any mass in the perineum or soft tissue of the pelvis. Long-term follow-up is necessary for early diagnosis of local recurrence.Introduction
Aggressive angiomyxoma (AA) is a rare, slow-growing locally infiltrative mesenchymal neoplasm that has a predilection for the pelvis and perineal regions. This neoplasm is seen more frequently in reproductive age group in females, the female to male ratio being 6:1. It is benign in nature but has a tendency for local recurrence. We present a report of this tumor occurring in a young female with systemic lupus erythematosus.
Case Report
A 26-year-old unmarried female presented with marked swelling of both labia majora with ulceration and foul-smelling discharge since 5 years. The swelling started as a small asymptomatic nodule over both labia majora. This gradually increased in size over the next 5 years to attain the presenting size. There was no menstrual complaints or difficulty in micturition or defecation. Since 6 months there was spontaneous development of superficial erosions with a scanty foul smelling sticky discharge. She had developed some difficulty in walking because of the huge size of the swelling. The patient had neither revealed her problem for 5 years to anyone including her mother nor sought medical advice as she was embarrassed about the abnormality on her genitalia.
She also gave a history of malar rash associated with photosensitivity and diffuse hair loss for the past 2 years. She had developed erythematous asymptomatic macules on the chest, back, and extremities which gradually turned hyperpigmented. She had history of recurrent episodes of fever and joint pain in the shoulders, ankles, and interphalangeal joints associated with mild swelling and minimal restriction of movements. She also had mild weakness in proximal upper limb muscles. A history of dyspnea on exertion was present since 6 months without associated cough or chest pain. There was no history of loss of weight or appetite, Raynaud′s phenomenon, digital or oral ulceration, or abdominal pain. There was no history of sexual exposure. Before presenting to us, she had been diagnosed as a case of systemic lupus erythematosus (SLE) for a year and treated with hydroxychloroquine, tapering doses of prednisolone from 40 to 10 mg daily and azathioprine for her skin lesions and joint pain with about 75% improvement in the symptoms except the genital swelling.
Except for mild pallor, her general and systemic examination was unremarkable. On cutaneous examination, multiple hyperpigmented macules of size varying from 2 × 2 cm to 5 × 5 cm were present over the face, neck, and upper limbs suggestive of subsiding lesions of lupus erythematosus. Some lesions also had minimal erythema. She had thin, sparse, and short scalp hair. The oral mucosa was normal. On examination of the genitalia, she had very large fusiform smooth looking swellings of both labia majora [Figure - 1] which extended up to the lower third of thigh in standing position. The skin over the swelling had about 4 × 1.5 cm size erosions at four places over the dependent area. These erosions were nontender and without background induration. The swelling was diffuse, smooth, nontender, and had a variable consistency from soft to firm in certain areas. There was no nodularity. There was no local or generalized lymphadenopathy. A per vaginal examination was not done as she was unmarried. A diagnosis of subsiding/almost subsided lupus erythematosus was considered for the skin lesions. The clinical differential diagnoses considered for the swelling of the labia majora were vascular malformation, soft tissue tumor, filariasis, and esthiomene.
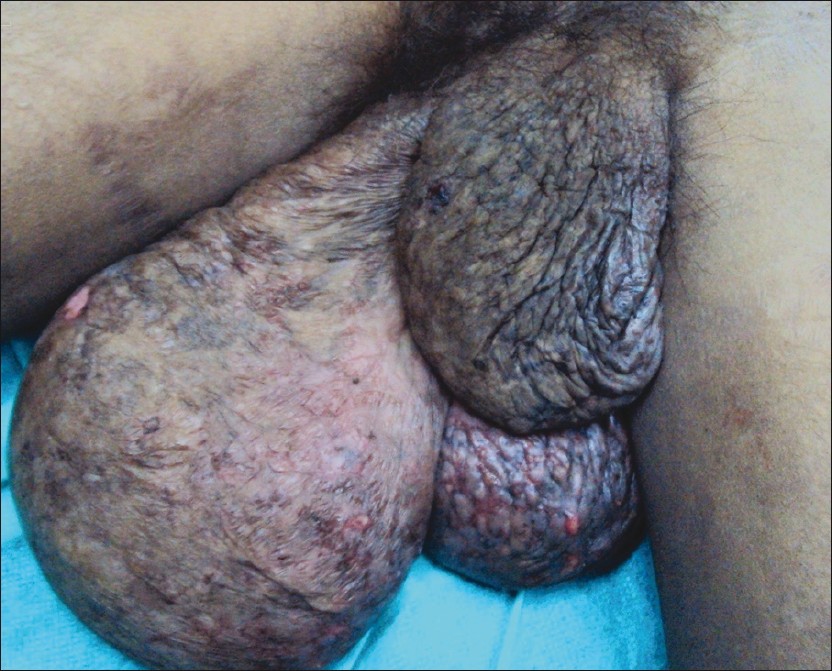 |
| Figure 1: Large fusiform smooth - looking swellings of both labia majora |
The hemogram revealed normochromic normocytic anemia with hemoglobin of 9.3 gm/dl. The liver and renal function tests were within normal limits. The ANA titer was raised (1:320 homogenous). The dsDNA titer was 1:20. There was no proteinuria on urine examination. Urine culture was sterile. From the erosions, the smears were made and Gram stain, Giemsa stain, and dark ground microscopy from the erosions did not reveal any organism. The Mantoux test, peripheral blood smear for microfilaria, and HIV serology were negative. VDRL was nonreactive. The pulmonary function tests revealed mild changes suggestive of restrictive lung disease. The high-resolution CT chest showed minimal bilateral pleural effusion. Ultrasound of the abdomen and pelvis revealed a fatty liver with cholelithiasis; the uterus, ovaries, and fallopian tubes appeared normal. Ultrasound of the genital swelling revealed marked edematous soft tissue; the vascularity was normal and no collection of fluid was seen. Fine needle aspiration cytology from the swelling yielded clear fluid which on microscopy showed few red blood cells on a proteinaceous background. No parasites were identified. Two skin biopsies were taken from the swelling which showed mild acanthosis of epidermis with sclerosis of dermis and pigment incontinence. Biopsy from one of the macules on the skin revealed melanophages in the upper dermis and mild chronic inflammatory infiltrate around the peri appendageal and perivascular location. At this point on gynecology consultation, excision of the entire vulval mass was planned as it was causing definite difficulty in walking and severe psychological distress. Excision of both masses was done under general anesthesia and sent for histopathology. On gross examination, the cut surface showed a well-demarcated yellowish gray glistening homogenous unencapsulated tumor reaching almost up to the overlying skin [Figure - 2]. On histopathological examination, bland spindle and stellate-shaped cells were seen in a myxoid matrix. These cells had eosinophilic cytoplasm and did not have nuclear pleomorphism or mitosis. Many thick-walled and thin-walled blood vessels with a perivascular lymphocytic infiltrate were also seen [Figure - 3].
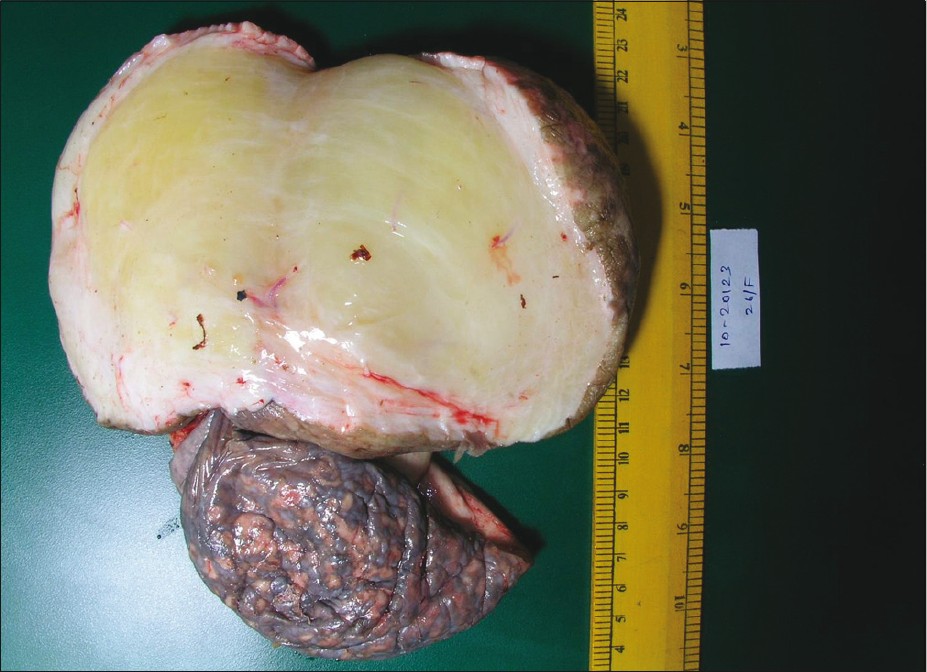 |
| Figure 2: Cut surface of the excised specimen showing a well-demarcated yellowish gray glistening homogenous unencapsulated tumor |
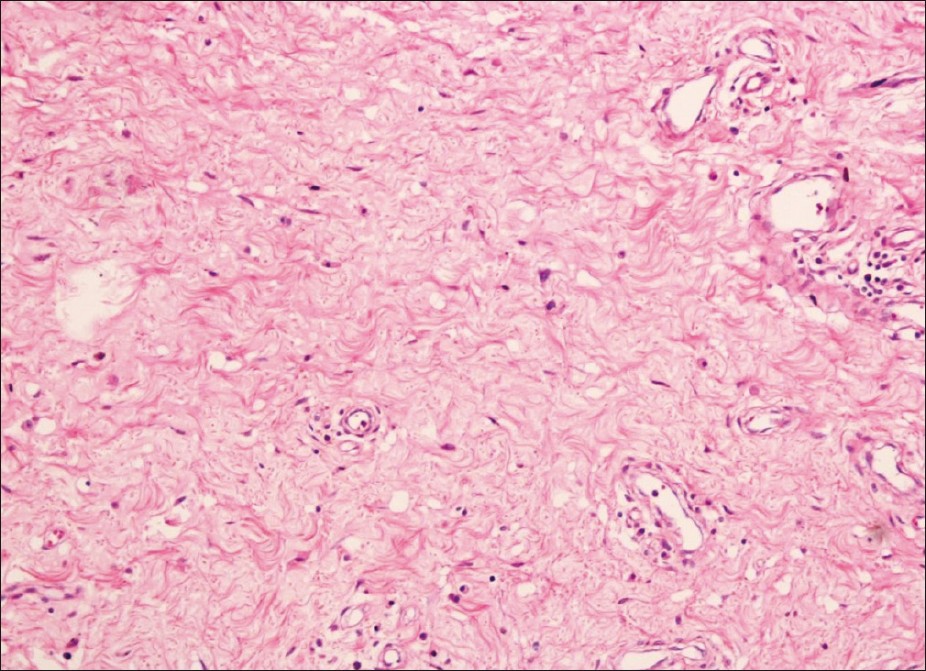 |
| Figure 3: Bland spindle and stellate-shaped cells were seen in a myxoid matrix. These cells had eosinophilic cytoplasm and did not have nuclear pleomorphism or mitosis.Various thick-walled and thin - walled blood vessels with a perivascular lymphocytic infiltrate were also seen (H and E, ×400) |
The recovery from surgery was uneventful. She was given sunscreens, tablet prednisolone 20 mg daily for 2 weeks and then tapered to 10 mg for 2 weeks and tablet hydroxycholoroqine 200 mg twice daily, with which the skin lesions of SLE further subsided. On follow-up for a year she was asymptomatic with no evidence of recurrence and scar and the vulval area was healthy [Figure - 4].
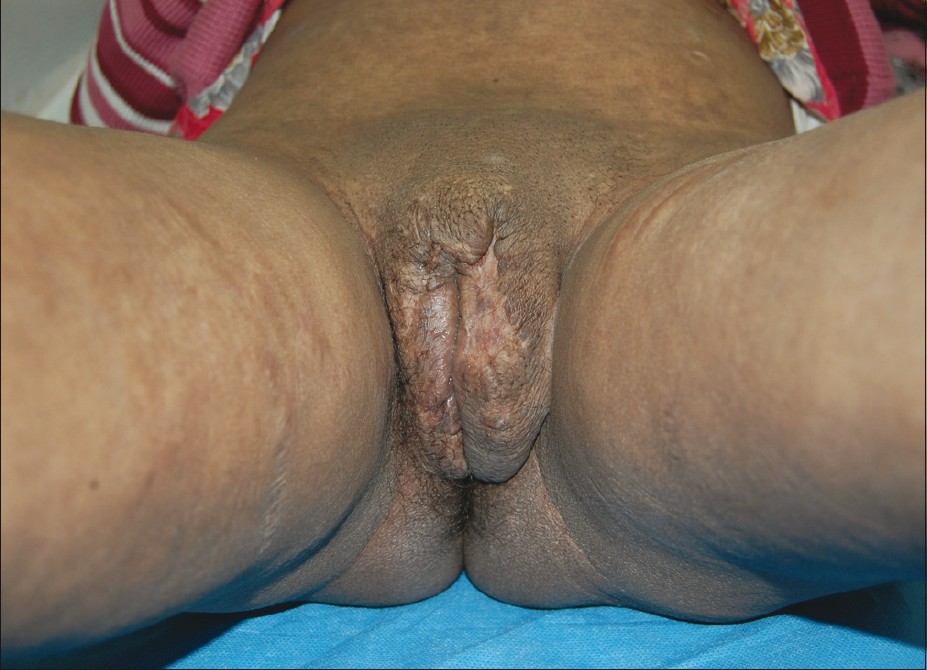 |
| Figure 4: Post - treatment photograph after 1 - year follow - up |
Discussion
AA, first described by Steeper and Rosai in 1983, [1] is a rare mesenchymal tumor of pelvic soft tissues seen predominantly in females. It has a peak incidence in the reproductive age group, although cases have been reported from 16 to 70 years. [2] It generally involves the genital, perineal, and pelvic region, with vulva being the most common site. Patients present with a painless, slowly growing soft tissue mass in the pelvi-perineal region, which may reach a large size by the time the patient seeks medical advice. Our case also had a similar course. A minority of cases have been reported in males, occurring in the scrotal, perineal, and inguinal regions. [3]
This tumor arises from fibroblasts or myoblasts. Microscopically, the tumor is composed of spindle and stellate-shaped cells in a myxoid matrix. The tumor cells express vimentin, desmin, and smooth muscle antigne (SMA) and are negative for S-100. [3] Angiomyxoma is termed aggressive because of its propensity for local recurrences. [4] Cytogenetic and molecular genetic analyses have revealed rearrangements of chromosomal region 12q13-15, resulting in altered expression of the HMGIC gene, a transcription factor belonging to the high mobility group of proteins. [3]
The differential diagnosis of aggressive angiomxyoma includes fibroepithelial stromal polyp, angiomyofibroblastoma, myxomas, superficial angiomyxoma, myxoid neurofibroma, myxoid liposarcoma, and myxofibrosarcoma. Superficial angiomyxoma differs clinically as they are superficially located and often polypoid. Histologically, they commonly involve the dermis and subcutaneous tissue, unlike AA, which is more deeply located.
Wide local excision is the therapy of choice. The excision of these tumors is difficult as they have the same consistency as that of normal connective tissue and therefore have a propensity for local recurrence. Systemic metastases have been reported to occur. [5] Long-term follow-up is necessary for early diagnosis of local recurrence and metastases. Imaging studies such as CT scan and MRI help in preoperative evaluation and postoperative follow-up as this tumor is ill defined clinically. [6] The presence of estrogen receptors in the tumor and its enlargement in pregnancy suggest the possibility of hormone dependence of this neoplasm. Gonadotropin-releasing hormone agonists have been suggested for those cases that are not amenable to surgical excision. [7]
The association of AA with SLE is not known. However, thymoma, [8] Hodgkin′s lymphoma, [9] monoclonal gammopathy and multiple myeloma, [10] and lymphatic leukemia are some neoplastic conditions that have been reported in patients having systemic lupus erythematosus. We could not find any basis of association between SLE and AA and in our patient SLE may just be a coincidence.
Initially, in our case the histopathology from the skin did not reveal any tumor as we may not have able to reach the depth of the tumor with the 4-mm skin punch. A definite diagnosis was possible only after the swelling was excised. Such cases may present to a dermatologist and AA should be considered as a differential diagnosis for any mass in the perineum or soft tissue of the pelvis, particularly the vulval mass. A regular follow-up is important to evaluate local recurrence, if any. Since the patient is still on hydroxychloroquine after 1 year of follow-up and initially she also received daily corticosteroids, these agents may or may not have some role in prevention of local recurrence. The excision definitely helped in marked improvement in quality of life.
| 1. |
Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum: Report of nine cases of a distinctive type of gynecologic soft tissue neoplasm. Am J Surg Pathol 1983;7:463-75.
[Google Scholar]
|
| 2. |
Fetsch JF, Laskin WB, Lefkowitz M, Kindblom LG, Meis-Kindblom JM. Aggressive angiomyxoma-a clinico pathological study of 29 female patients. Cancer 1996;78:79-90.
[Google Scholar]
|
| 3. |
Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology 1999;35:291-312.
[Google Scholar]
|
| 4. |
Rai L, Nandyala V, Shetty J, Kumar V, Rao L. Aggressive angiomyxoma: An important differential diagnosis for a vaginal mass. Aust N Z J Obstet Gynaecol 2004;44:367-8.
[Google Scholar]
|
| 5. |
Blandamura S, Cruz J, Faure Vergara L, Machado Puerto I, Ninfo V. Aggressive angiomyxoma: A second case of metastasis with patient's death. Hum Pathol 2003;34:1072-4.
[Google Scholar]
|
| 6. |
Outwater EK, Marchetto BE, Wagner BJ, Siegelman ES. Aggressive angiomyxoma: Findings on CT and MR imaging. AJR Am J Roentgenol 1999;172:435-8.
[Google Scholar]
|
| 7. |
McCluggage WG, Jamieson T, Dobbs SP, Grey A. Aggressive angiomyxoma of the vulva: Dramatic response to gonadotropin-releasing hormone agonist therapy. Gynecol Oncol 2006;100:623-5.
[Google Scholar]
|
| 8. |
Steven MM, Westedt ML, Eulderink F, Hazevoet HM, Dijkman JH, Cats A. Systemic lupus erythematosus and invasive thymoma: Report of two cases. Ann Rheum Dis 1984;43:25-8.
[Google Scholar]
|
| 9. |
Green JA, Dawson AA, Walker W. Systemic lupus erythematosus and lymphoma. Lancet 1978;312:753-6.
[Google Scholar]
|
| 10. |
Powell FC, Greipp PR, Su WP. Discoid lupus erythematosus and monoclonal gammopathy. Br J Dermatol 1983;109:355-60.
[Google Scholar]
|
Fulltext Views
2,656
PDF downloads
2,112





